An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonist/antagonists (ERAAs), are a class of drugs that act on the estrogen receptor (ER). A characteristic that distinguishes these substances from pure ER agonists and antagonists is that their action is different in various tissues, thereby granting the possibility to selectively inhibit or stimulate estrogen-like action in various tissues.

Selective Androgen Receptor Modulators or SARMs are a class of androgen receptor ligands that maintain some of the desirable effects of androgens, such as preventing osteoporosis and muscle loss while reducing risks of developing prostate cancer. In the late 1990s, the first nonsteroidal SARM, an analog of bicalutamide, was discovered. They are intended to have the same kind of effects as androgenic drugs, such as anabolic-androgenic steroids, but be more selective in their action. At present, there are no SARMs which have been approved for therapeutic use by the U.S. Food and Drug Administration.

BMS-564,929 is an investigational selective androgen receptor modulator (SARM) which is being developed by Bristol-Myers Squibb for treatment of the symptoms of age-related decline in androgen levels in men ("andropause"). These symptoms may include depression, loss of muscle mass and strength, reduction in libido and osteoporosis. Treatment with exogenous testosterone is effective in counteracting these symptoms but is associated with a range of side effects, the most serious of which is enlargement of the prostate gland, which can lead to benign prostatic hypertrophy and even prostate cancer. This means there is a clinical need for selective androgen receptor modulators, which produce anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in the prostate.

S-40503 is an investigational selective androgen receptor modulator (SARM) developed by the Japanese company Kaken Pharmaceuticals, which was developed for the treatment of osteoporosis. SARMs are a new class of drugs which produce tissue-specific anabolic effects in some tissues such as muscle and bone, but without stimulating androgen receptors in other tissues such as in the prostate gland, thus avoiding side effects such as benign prostatic hypertrophy which can occur following treatment with unselective androgens like testosterone or anabolic steroids.

LGD-2226 is an investigational selective androgen receptor modulator (SARM), which is being developed for treatment of muscle wasting and osteoporosis.

Enobosarm, also known as ostarine or MK-2866, is an investigational selective androgen receptor modulator (SARM) developed by GTx, Inc. for the treatment of conditions such as muscle wasting and osteoporosis, formerly under development by Merck & Company.
Andarine is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc for treatment of conditions such as muscle wasting, osteoporosis and benign prostatic hypertrophy, using the nonsteroidal antiandrogen bicalutamide as a lead compound.

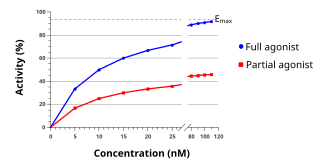
AC-262536 is a drug developed by Acadia Pharmaceuticals which acts as a selective androgen receptor modulator (SARM). Chemically it possesses endo-exo isomerism, with the endo form being the active form. It acts as a partial agonist for the androgen receptor with a Ki of 5nM, and no significant affinity for any other receptors tested. In animal studies it produced a maximal effect of around 66% of the anabolic action of testosterone, but only around 27% of its potency as an androgen.
This article is about the discovery and development of antiandrogens, or androgen receptor (AR) antagonists.

Ligandrol is a novel nonsteroidal oral selective androgen receptor modulator (SARM) for treatment of conditions such as muscle wasting and osteoporosis, discovered by Ligand Pharmaceuticals and under development by Viking Therapeutics.

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.
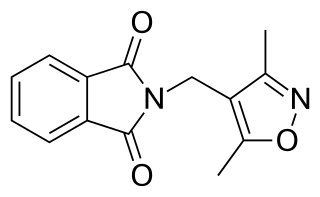
DIMP, or N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide, is a nonsteroidal antiandrogen (NSAA) structurally related to thalidomide that was first described in 1973 and was never marketed. Along with flutamide, it was one of the earliest NSAAs to be discovered, and for this reason, has been described as a "classical" NSAA. The drug is a selective, competitive, silent antagonist of the AR, although it is described as an "only relatively weak competitor". Its relative binding affinity for the androgen receptor is about 2.6% of that of metribolone. DIMP possesses no androgenic, estrogenic, progestogenic, or antigonadotropic activity, but it does reverse the antigonadotropic effects of testosterone, indicating that, like other pure AR antagonists, it is progonadotropic.

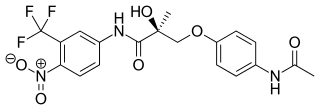
Acetothiolutamide is a selective androgen receptor modulator (SARM) derived from the nonsteroidal antiandrogen bicalutamide that was described in 2002 and was one of the first SARMs to be discovered and developed. It is a high-affinity, selective ligand of the androgen receptor (AR), where it acts as a full agonist in vitro, and has in vitro potency comparable to that of testosterone. However, in vivo, acetothiolutamide displayed overall negligible androgenic effects, though significant anabolic effects were observed at high doses. In addition, notable antiandrogen effects were observed in castrated male rats treated with testosterone propionate. The discrepancy between the in vitro and in vivo actions of acetothiolutamide was determined to be related to rapid plasma clearance and extensive hepatic metabolism into a variety of metabolites with differing pharmacological activity, including AR partial agonism and antagonism. In accordance with its poor metabolic stability, acetothiolutamide is not orally bioavailable, and shows activity only via injected routes such as subcutaneous and intravenous.

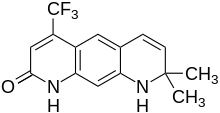
LG121071 is a selective androgen receptor modulator (SARM) developed by Ligand Pharmaceuticals that was first described in 1999 and was the first orally active nonsteroidal androgen to be discovered. It is a tricyclic quinolone derivative, structurally distinct from other nonsteroidal AR agonists like andarine and enobosarm (ostarine). The drug acts as a high-affinity full agonist of the androgen receptor (AR), with a potency and efficacy that is said to be equivalent to that of dihydrotestosterone (DHT). Unlike testosterone, but similarly to DHT, LG121071 and other nonsteroidal androgens cannot be potentiated by 5α-reductase in androgenic tissues, and for this reason, show tissue-selective androgenic effects. In accordance, they are said to possess full anabolic activity with reduced androgenic activity, similarly to anabolic-androgenic steroids.

5N-Bicalutamide, or 5-azabicalutamide, is a highly potent nonsteroidal antiandrogen (NSAA) which was discovered in 2016. It is a structural modification of bicalutamide differing it from it only by the replacement of a carbon atom with a nitrogen atom in one of its phenyl rings. Similarly to bicalutamide, the drug acts as a selective antagonist of the androgen receptor (AR). However, unlike bicalutamide, it is a reversible covalent antagonist and stays bound to the receptor for a far longer amount of time. As a result of this difference, 5N-bicalutamide has markedly improved potency relative to bicalutamide, with approximately 150-fold higher affinity for the AR (Ki = 0.15 nM versus 22.3 nM) and about 20-fold greater functional inhibition (IC50 = 15 nM versus 310 nM) of the AR. Future studies of 5N-bicalutamide in normal and mutated prostate cancer cells are planned or underway and it is anticipated that N-bicalutamide may be able to overcome resistance to current antiandrogens that are used in the treatment of prostate cancer.

RD-162 is a second-generation nonsteroidal antiandrogen (NSAA) which was developed for the treatment of prostate cancer but was never marketed. It acts as a potent and selective silent antagonist of the androgen receptor (AR). The drug is a diarylthiohydantoin derivative. It is closely related to enzalutamide and apalutamide. Both RD-162 and enzalutamide show 5- to 8-fold higher affinity for the AR than the first-generation NSAA bicalutamide, and only 2- to 3-fold lower affinity than dihydrotestosterone (DHT), the major endogenous ligand of the receptor in the prostate gland.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.

EM-5854 is a steroidal antiandrogen which was under development by Endoceutics, Inc. for the treatment of prostate cancer. It was first described in a patent in 2008, and was further characterized in 2012. EM-5854 reached phase I/II clinical trials for the treatment of prostate cancer but development was discontinued in March 2019.

GSK4336A is a drug which acts as a selective androgen receptor modulator (SARM), and was developed for androgen replacement therapy.