Mifepristone, also known as RU-486, is a medication typically used in combination with misoprostol to bring about an abortion during pregnancy. This combination is 97% effective during the first 63 days of pregnancy. It is also effective in the second trimester of pregnancy. Effectiveness should be verified two weeks after use. It is taken by mouth.

The progesterone receptor (PR), also known as NR3C3 or nuclear receptor subfamily 3, group C, member 3, is a protein found inside cells. It is activated by the steroid hormone progesterone.
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

A selective progesterone receptor modulator (SPRM) is an agent that acts on the progesterone receptor (PR), the biological target of progestogens like progesterone. A characteristic that distinguishes such substances from full receptor agonists and full antagonists is that their action differs in different tissues, i.e. agonist in some tissues while antagonist in others. This mixed profile of action leads to stimulation or inhibition in tissue-specific manner, which further raises the possibility of dissociating undesirable adverse effects from the development of synthetic PR-modulator drug candidates.
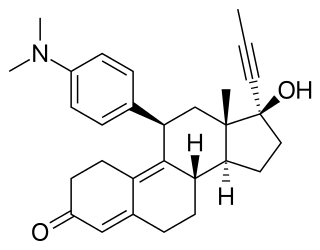
Asoprisnil is a synthetic, steroidal selective progesterone receptor modulator that was under development by Schering and TAP Pharmaceutical Products for the treatment of uterine fibroids. In 2005, phase III clinical trials were discontinued due to endometrial changes in patients.

Antiprogestogens, or antiprogestins, also known as progesterone antagonists or progesterone blockers, are a class of drugs which prevent progestogens like progesterone from mediating their biological effects in the body. They act by blocking the progesterone receptor (PR) and/or inhibiting or suppressing progestogen production. Antiprogestogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiandrogens.

Ulipristal acetate, sold under the brand name Ella among others, is a medication used for emergency contraception and uterine fibroids. As emergency contraception it should be used within 120 hours of sex. For fibroids it may be taken for up to six months. It is taken by mouth.

Aglepristone (INN) is a synthetic, steroidal antiprogestogen related to mifepristone which is marketed by Virbac in several European countries for use in veterinary medicine. It is specifically used as an abortifacient in pregnant animals. Aglepristone, similarly to mifepristone, also possesses some antiglucocorticoid activity.

Anordrin, also known as 2α,17α-diethynyl-A-nor-5α-androstane-2β,17β-diol dipropionate, is a synthetic, steroidal selective estrogen receptor modulator (SERM) which is used in China as an emergency contraceptive. It is the most commonly used emergency contraceptive in China. The drug is marketed in a combination formulation with mifepristone under the brand name Zi Yun. Anordrin has not been studied for use or marketed outside of China. It has been used in China since the 1970s.
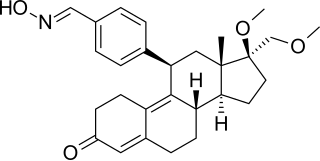
Onapristone (INN) is a synthetic and steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and described in 1984 but was never marketed. It is a silent antagonist of the progesterone receptor (PR), in contrast to the related antiprogestogen mifepristone. Moreover, compared to mifepristone, onapristone has reduced antiglucocorticoid activity, shows little antiandrogenic activity, and has 10- to 30-fold greater potency as an antiprogestogen. The medication was under development for clinical use, for instance in the treatment of breast cancer and as an endometrial contraceptive, but was discontinued during phase III clinical trials in 1995 due to findings that liver function abnormalities developed in a majority patients.

Toripristone (INN) is a synthetic, steroidal antiglucocorticoid as well as antiprogestogen which was never marketed. It is reported as a potent and highly selective antagonist of the glucocorticoid receptor (GR), though it also acts as an antagonist of the progesterone receptor (PR). The pharmacological profile of toripristone is said to be very similar to that of mifepristone, except that toripristone does not bind to orosomucoid. The drug has been used to study the hypothalamic-pituitary-adrenal axis and has been used as a radiotracer for the GR. Its INN was given in 1990.

Lilopristone (INN) is a synthetic, steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and was patented in 1985. It is described as an abortifacient and endometrial contraceptive. The drug differs from mifepristone only in the structure of its C17α side chain, and is said to have much reduced antiglucocorticoid activity in comparison.

Vilaprisan is a synthetic and steroidal selective progesterone receptor modulator (SPRM) which is under development by Bayer HealthCare Pharmaceuticals for the treatment of endometriosis and uterine fibroids. It is a potent and highly selective partial agonist of the progesterone receptor (PR). As of 2017, the drug is in phase II clinical trials for the aforementioned indications.
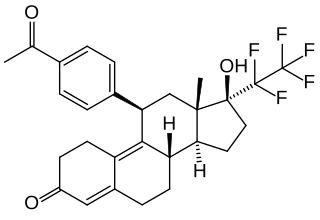
Lonaprisan is a synthetic, steroidal antiprogestogen which was under development by Bayer HealthCare Pharmaceuticals for the treatment of endometriosis, dysmenorrhea, and breast cancer but was discontinued. It is a potent and highly selective silent antagonist of the progesterone receptor (PR). The drug reached phase II clinical trials prior to its discontinuation.

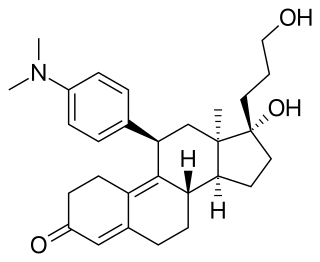
Asoprisnil ecamate (INN) is a synthetic, steroidal selective progesterone receptor modulator (SPRM) which was under development for the treatment of endometriosis, uterine fibroids, and menopausal symptoms but was discontinued. It is a potent and highly selective ligand of the progesterone receptor with mixed agonistic and antagonistic activity and much reduced antiglucocorticoid activity relative to mifepristone. The drug reached phase III clinical trials for the aforementioned indications prior to its discontinuation.

5α-Dihydronorethisterone is a major active metabolite of norethisterone (norethindrone). Norethisterone is a progestin with additional weak androgenic and estrogenic activity. 5α-DHNET is formed from norethisterone by 5α-reductase in the liver and other tissues.
A sex-hormonal agent, also known as a sex-hormone receptor modulator, is a type of hormonal agent which specifically modulates the effects of sex hormones and of their biological targets, the sex hormone receptors. The sex hormones include androgens such as testosterone, estrogens such as estradiol, and progestogens such as progesterone. Sex-hormonal agents may be either steroidal or nonsteroidal in chemical structure and may serve to either enhance, inhibit, or have mixed effects on the function of the sex hormone systems.

EM-5854 is a steroidal antiandrogen which was under development by Endoceutics, Inc. for the treatment of prostate cancer. It was first described in a patent in 2008, and was further characterized in 2012. EM-5854 reached phase I/II clinical trials for the treatment of prostate cancer but development was discontinued in March 2019.

Metapristone is the major metabolite of mifepristone and a selective progesterone receptor modulator (SPRM) which itself was never marketed. It is formed from mifepristone in the liver by the enzyme CYP3A4 via monodemethylation, and circulates at concentrations higher than those of mifepristone. The metabolite retains partial but considerable affinity for the progesterone receptor (PR) and the glucocorticoid receptor (GR). On the basis of actions that are apparently independent of its hormonal activity, metapristone is being researched as a potential cancer metastatic chemopreventive agent.
















