Sodium ferulate (SF), the sodium salt of ferulic acid, is a drug used in traditional Chinese medicine for treatment of cardiovascular and cerebrovascular diseases and to prevent thrombosis. It is found in the root of Angelica sinensis. It is considered safe and effective. Ferulic acid can also be extracted from the root of the Chinese herb Ligusticum chuanxiong.

Ligusticum is a genus of about 60 species of flowering plants in the family Apiaceae, native to cool temperate regions of the Northern Hemisphere. Its name is believed to derive from the Italian region of Liguria.

Aralkylamine N-acetyltransferase (AANAT), also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb AANAT gene containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80% identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of N-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.

Lofentanil is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.
Ligusticum striatum is a flowering plant in the carrot family best known for its use in traditional Chinese medicine where it is considered one of the 50 fundamental herbs. It is known by the common name Szechuan lovage, and chuānxiōng in Chinese: 川芎. It is native to India, Kashmir, and Nepal. It contains the phytoprogestogens 3,8-dihydrodiligustilide and riligustilide.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

YM-348 is an indazole derivative drug which acts as a potent and selective 5-HT2C receptor agonist, with an EC50 of 1nM and 15x selectivity over 5-HT2A, although it only has moderate selectivity of 3x over the closely related 5-HT2B receptor. It has thermogenic and anorectic effects in animal studies, making it potentially useful for the treatment of obesity.

SB-269970 is a drug and research chemical developed by GlaxoSmithKline used in scientific studies. It is believed to act as a selective 5-HT7 receptor antagonist (EC50 = 1.25 nM) (or possibly inverse agonist). A subsequent study in guinea pig at 10 uM showed that it also blocks the α2-adrenergic receptor activity. The significant difference in test concentrations, however, confirms the selectivity of SB-269970 for the 5-HT7 receptor.

Harmane (harman) is a heterocyclic amine found in a variety of foods including coffee, sauces, and cooked meat. It is also present in tobacco smoke.
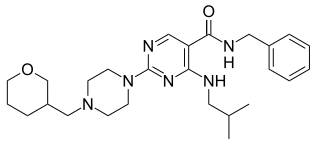
PF-4840154 is pyrimidine derivative discovered by Pfizer at its Sandwich, Kent research center. The compound is a potent, selective activator of both the human (EC50 = 23 nM) and rat (EC50 = 97 nM) TRPA1 channels. This compound elicits nociception in a mouse model through TRPA1 activation. PF-4840154 is used as a reference agonist of the TRPA1 channel for in-vitro high-throughput screening purposes, and is superior to allyl isothiocyanate for this use. The TRPA1 channel is considered an attractive pain target based on the fact that TRPA1 knockout mice showed near complete attenuation of pain behaviors in some pre-clinical development models.

AB-001 or 1-pentyl-3-(1-adamantoyl)indole is a designer drug that was found as an ingredient in synthetic cannabis smoking blends in Ireland in 2010 and Hungary and Germany in 2011. It is unclear who AB-001 was originally developed by, but it is structurally related to compounds such as AM-1248 and its corresponding 1-(tetrahydropyran-4-ylmethyl) analogue, which are known to be potent cannabinoid agonists with moderate to high selectivity for CB2 over CB1. The first published synthesis and pharmacological evaluation of AB-001 revealed that it acts as a full agonist at CB1 (EC50 = 35 nM) and CB2 receptors (EC50 = 48 nM). However, AB-001 was found to possess only weak cannabimimetic effects in rats at doses up to 30 mg/kg, making it less potent than the carboxamide analogue APICA, which possesses potent cannabimimetic activity at doses of 3 mg/kg.

WAY-161503 is a full agonist of 5-HT2C receptors (Ki = 3.3 nM for displacement of DOI), ~6-fold less potent at 5-HT2A receptors (Ki = 18 nM) and 20-fold less potent at 5-HT2B receptors (Ki = 60 nM). In functional studies, it stimulates calcium mobilization coupled to 5-HT2C, 5-HT2B, and 5-HT2A receptors with EC50 values of 0.8, 1.8, and 7 nM, respectively. WAY-161503 has been reported to produce dose-dependent decreases in food intake in 24-hour fasted normal Sprague-Dawley rats, diet-induced obese mice, and obese Zucker rats with ED50 values of 1.9, 6.8, and 0.73 mg/kg, respectively.

APICA is an indole based drug that acts as a potent agonist for the cannabinoid receptors.
4-Nonylphenylboronic acid is a potent and selective inhibitor of the enzyme fatty acid amide hydrolase (FAAH), with an IC50 of 9.1nM, and 870x selectivity for FAAH over the related enzyme MAGL, which it inhibits with an IC50 of 7900nM. It is also a weaker inhibitor of the enzymes endothelial lipase and lipoprotein lipase, with IC50 values of 100nM and 1400nM respectively.

Cebranopadol is a novel opioid analgesic of the benzenoid class which is currently under development internationally by Grünenthal, a German pharmaceutical company, and its partner Depomed, a pharmaceutical company in the United States, for the treatment of a variety of different acute and chronic pain states. As of November 2014, it is in phase III clinical trials.
Guineesine is an alkaloid isolated from long pepper and black pepper.

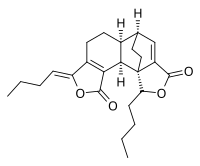
Riligustilide is a nonsteroidal phytoprogestogen that is found in Ligusticum chuanxiong. It is a very weak agonist of the progesterone receptor (EC50 ≈ 81 μM). Another compound in the plant, 3,8-dihydrodiligustilide, is also a phytoprogestogen, but is almost 1,000-fold more potent in comparison (EC50 = 90 nM).

3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol is a synthetic, steroidal estrogen and a selective agonist of the ERβ. It was discovered serendipitously and was the lead compound among a series of androsta-3,5-dienes as ERβ ligands. Its affinity (IC50) for the ERβ was found to be 9 nM and it showed 62- and 160-fold binding selectivity for this receptor over the AR and the ERα, respectively. The EC50 of the compound for the ERβ was found to be 69 nM and its intrinsic activity was 92% (relative to that of estradiol). As such, it is a potent ERβ agonist with high affinity and selectivity.