Related Research Articles

Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.
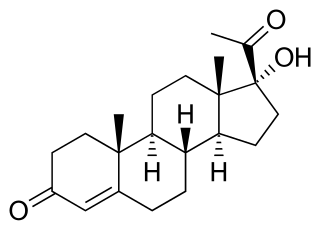
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.

Drospirenone is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy and in menopausal hormone therapy, among other uses. It is available both alone under the brand name Slynd and in combination with an estrogen under the brand name Yasmin among others. The medication is an analog of the drug spironolactone. Drospirenone is taken by mouth.

Norethisterone, also known as norethindrone and sold under many brand names, is a progestin medication used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. The medication is available in both low-dose and high-dose formulations and both alone and in combination with an estrogen. It is used by mouth or, as norethisterone enanthate, by injection into muscle.

Medroxyprogesterone (MP), is a progestin which is not used medically. A derivative, medroxyprogesterone acetate (MPA), is used as a medication in humans, and is far more widely known in comparison. Medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate, and what is almost always being referred to when the term is used is MPA and not medroxyprogesterone.
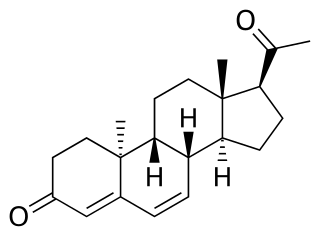
Dydrogesterone, sold under the brand name Dydroboon & Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Norgestrienone, sold under the brand names Ogyline, Planor, and Miniplanor, is a progestin medication which has been used in birth control pills, sometimes in combination with ethinylestradiol. It was developed by Roussel Uclaf and has been registered for use only in France. Under the brand name Planor, it has been marketed in France as 2 mg norgestrienone and 50 μg ethinylestradiol tablets. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.
Ligusticum striatum is a flowering plant native to India, Kashmir, and Nepal in the carrot family best known for its use in traditional Chinese medicine where it is considered one of the 50 fundamental herbs. It is known by the common name Szechuan Lovage. It contains the phytoprogestogens 3,8-dihydrodiligustilide and riligustilide.

Dienogest, sold under the brand name Visanne among others, is a progestin medication which is used in birth control pills and in the treatment of endometriosis. It is also used in menopausal hormone therapy and to treat heavy periods. Dienogest is available both alone and in combination with estrogens. It is taken by mouth.

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.
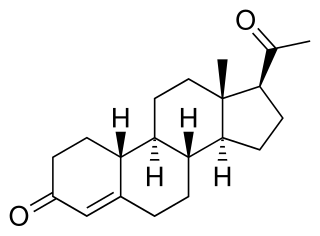
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.

5β-Dihydroprogesterone is an endogenous neurosteroid and an intermediate in the biosynthesis of pregnanolone and epipregnanolone from progesterone. It is synthesized from progesterone by the enzyme 5β-reductase.

3,8-Dihydrodiligustilide is a nonsteroidal phytoprogestogen that is found in Ligusticum chuanxiong. It is a potent agonist of the progesterone receptor (EC50 = 90 nM). Another compound in the plant, riligustilide, is also a phytoprogestogen, but is almost 1,000-fold less potent and is very weak in comparison (EC50 ≈ 81 μM).

Riligustilide is a nonsteroidal phytoprogestogen that is found in Ligusticum chuanxiong. It is a very weak agonist of the progesterone receptor (EC50 ≈ 81 μM). Another compound in the plant, 3,8-dihydrodiligustilide, is also a phytoprogestogen, but is almost 1,000-fold more potent in comparison (EC50 = 90 nM).
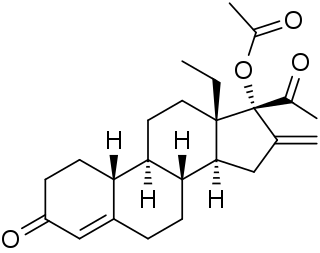
18-Methylsegesterone acetate is a progestin medication of the 19-norprogesterone group which was never marketed. It was first described in a patent in 1997 and then in a literature paper in 2003. 18-Methyl-SGA is the C18 methyl or C13β ethyl derivative of segesterone acetate, and shows 3 to 10 times the progestogenic potency of SGA in bioassays. This is analogous to the case of the 19-nortestosterone progestin norethisterone and its 18-methyl derivative levonorgestrel, the latter showing substantially increased potency relative to the former similarly. As SGA is already one of the most potent progestins to have been developed, with 100-fold the potency of progesterone and 10-fold the potency of levonorgestrel in bioassays, 18-methyl-SGA is an extremely potent progestogen, among if not the most potent known.
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.
References
- 1 2 3 Hajirahimkhan, Atieh; Dietz, Birgit; Bolton, Judy (2013). "Botanical Modulation of Menopausal Symptoms: Mechanisms of Action?". Planta Medica. 79 (7): 538–553. doi:10.1055/s-0032-1328187. ISSN 0032-0943. PMC 3800090 . PMID 23408273.
- 1 2 Zava DT, Dollbaum CM, Blen M (1998). "Estrogen and progestin bioactivity of foods, herbs, and spices". Proc. Soc. Exp. Biol. Med. 217 (3): 369–78. doi:10.3181/00379727-217-44247. PMID 9492350. S2CID 20673587.
- ↑ Toh, M.F.; Sohn, J.; Chen, S.N.; Yao, P.; Bolton, J.L.; Burdette, J.E. (2012). "Biological characterization of non-steroidal progestins from botanicals used for women's health". Steroids. 77 (7): 765–773. doi:10.1016/j.steroids.2012.03.013. ISSN 0039-128X. PMC 3601661 . PMID 22484153.
- ↑ Lim LS, Shen P, Gong YH, Yong EL (2006). "Dimeric progestins from rhizomes of Ligusticum chuanxiong". Phytochemistry. 67 (7): 728–34. Bibcode:2006PChem..67..728L. doi:10.1016/j.phytochem.2006.01.024. PMID 16516938.
- ↑ Ahmed, H.M.M.; Yeh, J.Y.; Lin, W.J.; Forsberg, N.E.; Cheng, W.T.K.; Ou, B.R (2014). "Validation of a luciferase bioassay to detect the progestative activity in gilts whose estrus was induced by an uterotonic herb (Ligusticum chuanxiong)". Livestock Science. 163: 159–164. doi:10.1016/j.livsci.2014.02.012. ISSN 1871-1413.