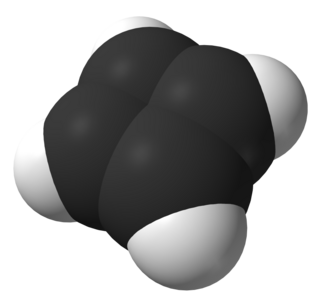
Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π-electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
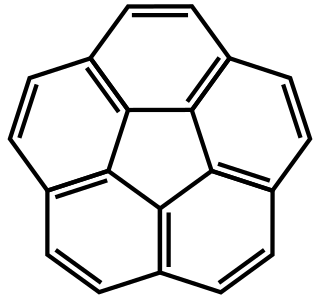
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.

Cyclooctadecanonaene or [18]annulene is an organic compound with chemical formula C
18H
18. It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that [18]annulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the cis,trans,trans,cis,trans,trans,cis,trans,trans configuration. It is reported to be a red-brown crystalline solid.

A persistent carbene is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet, but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC), in which nitrogen atoms flank the formal carbene.

An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field.
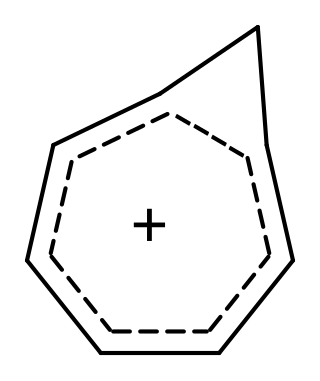
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

Tricyclobutabenzene is an aromatic hydrocarbon consisting of a benzene core with three cyclobutane rings fused onto it. This compound and related compounds are studied in the laboratory because they are often displaying unusual conformations and because of their unusual reactivity. Tricyclobutabenzenes are isomers of radialenes and form an equilibrium with them.
In organic chemistry, propellane is any member of a class of polycyclic hydrocarbons, whose carbon skeleton consists of three rings of carbon atoms sharing a common carbon–carbon covalent bond. The concept was introduced in 1966 by D. Ginsburg Propellanes with small cycles are highly strained and unstable, and are easily turned into polymers with interesting structures, such as staffanes. Partly for these reasons, they have been the object of much research.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.

Cyclododecahexaene or [12]annulene is a member of the series of annulenes with some interest in organic chemistry with regard to the study of aromaticity. Cyclododecahexaene is non-aromatic due to the lack of planarity of the structure. On the other hand the dianion with 14 electrons is a Hückel aromat and more stable.

A 4-center 2-electron (4c–2e) bond is a type of chemical bond in which four atoms share two electrons in bonding, with a net bond order of 1⁄2. This type of bonding differs from the usual covalent bond, which involves two atoms sharing two electrons.
Cyclotetradecaheptaene, often referred to as [14]annulene, is a hydrocarbon with molecular formula C14H14, which played an important role in the development of criteria (Hückel's rule) for aromaticity, a stabilizing property of central importance in physical organic chemistry. It forms dark-red needle-like crystals.
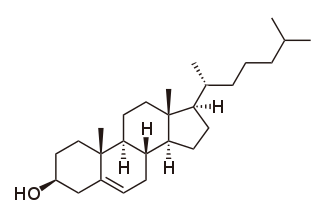
Cholesterol total synthesis in chemistry describes the total synthesis of the complex biomolecule cholesterol and is considered a great scientific achievement. The research group of Robert Robinson with John Cornforth published their synthesis in 1951 and that of Robert Burns Woodward with Franz Sondheimer in 1952. Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone were produced from natural resources but expensive. Chemical companies Merck & Co. and Monsanto saw commercial opportunities for steroid synthesis and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.

1,6-Methano[10]annulene (also known as 1,6-methanonaphthalene or homonaphthalene) is an aromatic hydrocarbon with chemical formula C11H10. It was the first stable aromatic compound based on the cyclodecapentaene system to be discovered.
In organic chemistry, the oxy-Cope rearrangement is a chemical reaction. It involves reorganization of the skeleton of certain unsaturated alcohols. It is a variation of the Cope rearrangement in which 1,5-dien-3-ols are converted to unsaturated carbonyl compounds by a mechanism typical for such a [3,3]-sigmatropic rearrangement.

![[8]Annulyne trimerization in presence of base Annulynetrimerization.png](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/Annulynetrimerization.png/300px-Annulynetrimerization.png)
![Proposed [12]Annulyne synthesis from 1,5-hexadiyne, Stevenson 2005 -12-annulene.png](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e0/-12-annulene.png/400px--12-annulene.png)













