
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell.
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

Kerogen is solid, insoluble organic matter in sedimentary rocks. It consists of a variety of organic materials, including dead plants, algae, and other microorganisms, that have been compressed and heated by geological processes. Altogether kerogen is estimated to contain 1016 tons of carbon. This makes it the most abundant source of organic compounds on earth, exceeding the total organic content of living matter 10,000-fold.

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.
In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces, i.e., one has at least partial negative charge and the partner has partial positive charge, referred to respectively as the electron acceptor and electron donor. In some cases, the degree of charge transfer is "complete", such that the CT complex can be classified as a salt. In other cases, the charge-transfer association is weak, and the interaction can be disrupted easily by polar solvents.
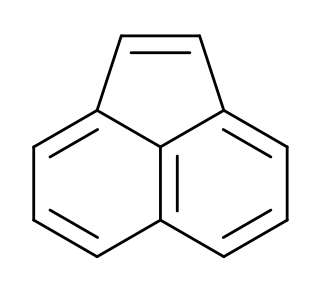
Acenaphthylene, a polycyclic aromatic hydrocarbon is an ortho- and peri-fused tricyclic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a -CH=CH- unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.

Acenaphthoquinone is a quinone derived from acenaphthene. It is a water-insoluble yellow solid. It is a precursor to some agrichemicals and dyes.
Aromatization is a chemical reaction in which an aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene. Aromatization includes the formation of heterocyclic systems.

Fluorene, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar. It is insoluble in water and soluble in many organic solvents. Although sometimes classified as a polycyclic aromatic hydrocarbon, the five-membered ring has no aromatic properties. Fluorene is mildly acidic.
1,2,4-Trimethylbenzene, also known as pseudocumene, is an organic compound with the chemical formula C6H3(CH3)3. Classified as an aromatic hydrocarbon, it is a flammable colorless liquid with a strong odor. It is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar and petroleum (about 3%). It is one of the three isomers of trimethylbenzene.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.
1,2,3-Trimethylbenzene is an organic compound with the chemical formula C6H3(CH3)3. Classified as an aromatic hydrocarbon, it is a flammable colorless liquid. It is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar and petroleum. It is one of the three isomers of trimethylbenzene. It is used in jet fuel, mixed with other hydrocarbons, to prevent the formation of solid particles which might damage the engine.

Isodurene or 1,2,3,5-tetramethylbenzene is an organic compound with the formula C6H2(CH3)4, classified as an aromatic hydrocarbon. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar. Isodurene is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene).
Pentamethylbenzene is an organic compound with the formula C6H(CH3)5. It is a colourless solid with a sweet odor. The compound is classified as an aromatic hydrocarbon. It is a relatively easily oxidized benzene derivative, with E1/2 of 1.95 V vs NHE.

An alkylbenzene is a chemical compound that contains a monocyclic aromatic ring attaching to one or more saturated hydrocarbon chains. Alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups. The simplest member, toluene, has the hydrogen atom of the benzene ring replaced by a methyl group. The chemical formula of alkylbenzenes is CnH2n-6.

o-Cymene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring ortho-substituted with a methyl group and an isopropyl group. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents.

m-Cymene is an organic compound classified as an aromatic hydrocarbon. Its structure consists of a benzene ring meta-substituted with a methyl group and an isopropyl group. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents.














