Related Research Articles

The Apicomplexa are organisms of a large phylum of mainly parasitic alveolates. Most possess a unique form of organelle structure that comprises a type of non-photosynthetic plastid called an apicoplast—with an apical complex membrane. The organelle's apical shape is an adaptation that the apicomplexan applies in penetrating a host cell.
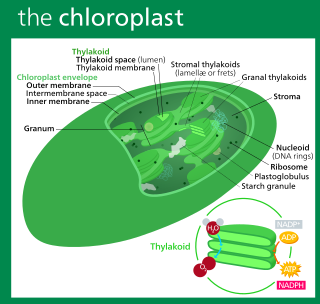
A chloroplast is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water in the cells. The ATP and NADPH is then used to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like Arabidopsis and wheat.
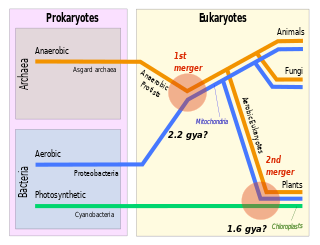
Symbiogenesis is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes taken one inside the other in endosymbiosis. Mitochondria appear to be phylogenetically related to Rickettsiales bacteria, while chloroplasts are thought to be related to cyanobacteria.

The alveolates are a group of protists, considered a major clade and superphylum within Eukarya. They are currently grouped with the stramenopiles and Rhizaria among the protists with tubulocristate mitochondria into the SAR supergroup.
A plastid is a membrane-bound organelle found in the cells of plants, algae, and some other eukaryotic organisms. Plastids are considered to be intracellular endosymbiotic cyanobacteria.

Chromista is a proposed but polyphyletic biological kingdom, refined from the Chromalveolata, consisting of single-celled and multicellular eukaryotic species that share similar features in their photosynthetic organelles (plastids). It includes all eukaryotes whose plastids contain chlorophyll c and are surrounded by four membranes. If the ancestor already possessed chloroplasts derived by endosymbiosis from red algae, all non-photosynthetic Chromista have secondarily lost the ability to photosynthesise. Its members might have arisen independently as separate evolutionary groups from the last eukaryotic common ancestor.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.

Plasmodium falciparum is a unicellular protozoan parasite of humans, and the deadliest species of Plasmodium that causes malaria in humans. The parasite is transmitted through the bite of a female Anopheles mosquito and causes the disease's most dangerous form, falciparum malaria. It is responsible for around 50% of all malaria cases. P. falciparum is therefore regarded as the deadliest parasite in humans. It is also associated with the development of blood cancer and is classified as a Group 2A (probable) carcinogen.

Merozoitesurface proteins are both integral and peripheral membrane proteins found on the surface of a merozoite, an early life cycle stage of a protozoan. Merozoite surface proteins, or MSPs, are important in understanding malaria, a disease caused by protozoans of the genus Plasmodium. During the asexual blood stage of its life cycle, the malaria parasite enters red blood cells to replicate itself, causing the classic symptoms of malaria. These surface protein complexes are involved in many interactions of the parasite with red blood cells and are therefore an important topic of study for scientists aiming to combat malaria.

Micronemes are secretory organelles, possessed by parasitic apicomplexans. Micronemes are located on the apical third of the protozoan body. They are surrounded by a typical unit membrane. On electron microscopy they have an electron-dense matrix due to the high protein content. They are specialized secretory organelles important for host-cell invasion and gliding motility.

A rhoptry is a specialized secretory organelle. They are club-shaped organelles connected by thin necks to the extreme apical pole of the parasite. These organelles, like micronemes, are characteristic of the motile stages of Apicomplexa protozoans. They can vary in number and shape and contain numerous enzymes that are released during the process of host penetration. The proteins they contain are important in the interaction between the host and the parasite, including the formation of the parasitophorous vacuole (PV).
Acidocalcisomes are rounded electron-dense acidic organelles, rich in calcium and polyphosphate and between 100 nm and 200 nm in diameter.
Neospora hughesi is an obligate protozoan apicomplexan parasite that causes myelitis and equine protozoal myeloencephalitis (EPM) in horses, and has only been documented in North America. EPM is a neurological disease from lesions in the spinal cord, brain stem, or brain from parasites such as N. hughesi or Sarcocystis neurona. Signs that a horse may have EPM include ataxia, muscle atrophy, difficulty swallowing, and head tilt. There are antiprotozoal drugs, such as the 28-day course of ponazuril, to treat the disease, as well as anti-inflammatories to alleviate neurologic symptoms
Chromera velia, also known as a "chromerid", is a unicellular photosynthetic organism in the superphylum Alveolata. It is of interest in the study of apicomplexan parasites, specifically their evolution and accordingly, their unique vulnerabilities to drugs.

Guillardia is a genus of marine biflagellate cryptomonad algae with a plastid obtained through secondary endosymbiosis of a red alga.
The N-end rule is a rule that governs the rate of protein degradation through recognition of the N-terminal residue of proteins. The rule states that the N-terminal amino acid of a protein determines its half-life. The rule applies to both eukaryotic and prokaryotic organisms, but with different strength, rules, and outcome. In eukaryotic cells, these N-terminal residues are recognized and targeted by ubiquitin ligases, mediating ubiquitination thereby marking the protein for degradation. The rule was initially discovered by Alexander Varshavsky and co-workers in 1986. However, only rough estimations of protein half-life can be deduced from this 'rule', as N-terminal amino acid modification can lead to variability and anomalies, whilst amino acid impact can also change from organism to organism. Other degradation signals, known as degrons, can also be found in sequence.

In molecular biology, Duffy binding proteins are found in Plasmodium. Plasmodium vivax and Plasmodium knowlesi merozoites invade Homo sapiens erythrocytes that express Duffy blood group surface determinants. The Duffy receptor family is localised in micronemes, an organelle found in all organisms of the phylum Apicomplexa.

The parasitophorous vacuole (PV) is a structure produced by apicomplexan parasites in the cells of its host. The PV allows the parasite to develop while protected from the phagolysosomes of the host cell.
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a family of proteins present on the membrane surface of red blood cells that are infected by the malarial parasite Plasmodium falciparum. PfEMP1 is synthesized during the parasite's blood stage inside the RBC, during which the clinical symptoms of falciparum malaria are manifested. Acting as both an antigen and adhesion protein, it is thought to play a key role in the high level of virulence associated with P. falciparum. It was discovered in 1984 when it was reported that infected RBCs had unusually large-sized cell membrane proteins, and these proteins had antibody-binding (antigenic) properties. An elusive protein, its chemical structure and molecular properties were revealed only after a decade, in 1995. It is now established that there is not one but a large family of PfEMP1 proteins, genetically regulated (encoded) by a group of about 60 genes called var. Each P. falciparum is able to switch on and off specific var genes to produce a functionally different protein, thereby evading the host's immune system. RBCs carrying PfEMP1 on their surface stick to endothelial cells, which facilitates further binding with uninfected RBCs, ultimately helping the parasite to both spread to other RBCs as well as bringing about the fatal symptoms of P. falciparum malaria.
A plastid is a membrane-bound organelle found in plants, algae and other eukaryotic organisms that contribute to the production of pigment molecules. Most plastids are photosynthetic, thus leading to color production and energy storage or production. There are many types of plastids in plants alone, but all plastids can be separated based on the number of times they have undergone endosymbiotic events. Currently there are three types of plastids; primary, secondary and tertiary. Endosymbiosis is reputed to have led to the evolution of eukaryotic organisms today, although the timeline is highly debated.
References
- 1 2 3 4 Lim L, McFadden GI (March 2010). "The evolution, metabolism and functions of the apicoplast". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 365 (1541): 749–63. doi:10.1098/rstb.2009.0273. PMC 2817234 . PMID 20124342.
- ↑ Sheiner L, Vaidya AB, McFadden GI (August 2013). "The metabolic roles of the endosymbiotic organelles of Toxoplasma and Plasmodium spp". Current Opinion in Microbiology. 16 (4): 452–8. doi:10.1016/j.mib.2013.07.003. PMC 3767399 . PMID 23927894.
- ↑ Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson RB (2011). Campbell Biology (9th ed.). Boston: Benjamin Cummings. ISBN 978-0-321-55823-7. OCLC 1008837408.
- 1 2 3 4 5 6 7 8 9 10 Maréchal E, Cesbron-Delauw MF (May 2001). "The apicoplast: a new member of the plastid family". Trends in Plant Science. 6 (5): 200–5. Bibcode:2001TPS.....6..200M. doi:10.1016/s1360-1385(01)01921-5. PMID 11335172.
- 1 2 3 4 5 Ralph SA, D'Ombrain MC, McFadden GI (June 2001). "The apicoplast as an antimalarial drug target". Drug Resistance Updates. 4 (3): 145–51. doi:10.1054/drup.2001.0205. PMID 11768328.
- ↑ Ralph SA, Foth BJ, Hall N, McFadden GI (December 2004). "Evolutionary pressures on apicoplast transit peptides". Molecular Biology and Evolution. 21 (12): 2183–94. doi: 10.1093/molbev/msh233 . PMID 15317876.
- ↑ Alberts B, Bray D, Hopkin K (2004). "The Eukaryotic Cell". Essential Cell Biology (2nd ed.). New York; London: Garland Science, Taylor & Francis Group. ISBN 978-0-8153-3480-4. OCLC 895254951.
- 1 2 3 4 5 6 Kimball JW (16 December 2017). "Endosymbiosis and The Origin of Eukaryotes". Kimball's Biology Pages.
- ↑ Sheiner L, Striepen B (February 2013). "Protein sorting in complex plastids". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833 (2): 352–9. doi:10.1016/j.bbamcr.2012.05.030. PMC 3494742 . PMID 22683761.
- ↑ Sheiner L, Fellows JD, Ovciarikova J, Brooks CF, Agrawal S, Holmes ZC, Bietz I, Flinner N, Heiny S, Mirus O, Przyborski JM, Striepen B (December 2015). "Toxoplasma gondii Toc75 Functions in Import of Stromal but not Peripheral Apicoplast Proteins" (PDF). Traffic. 16 (12): 1254–69. doi: 10.1111/tra.12333 . PMID 26381927.
- ↑ Agrawal S, van Dooren GG, Beatty WL, Striepen B (November 2009). "Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins". The Journal of Biological Chemistry. 284 (48): 33683–91. doi: 10.1074/jbc.M109.044024 . PMC 2785210 . PMID 19808683.
- ↑ Dahl EL, Shock JL, Shenai BR, Gut J, DeRisi JL, Rosenthal PJ (September 2006). "Tetracyclines specifically target the apicoplast of the malaria parasite Plasmodium falciparum". Antimicrobial Agents and Chemotherapy. 50 (9): 3124–31. doi:10.1128/AAC.00394-06. PMC 1563505 . PMID 16940111.