Biosafety is the prevention of large-scale loss of biological integrity, focusing both on ecology and human health. These prevention mechanisms include the conduction of regular reviews of biosafety in laboratory settings, as well as strict guidelines to follow. Biosafety is used to protect from harmful incidents. Many laboratories handling biohazards employ an ongoing risk management assessment and enforcement process for biosafety. Failures to follow such protocols can lead to increased risk of exposure to biohazards or pathogens. Human error and poor technique contribute to unnecessary exposure and compromise the best safeguards set into place for protection.

Biosecurity refers to measures aimed at preventing the introduction or spread of harmful organisms intentionally or unintentionally outside their native range or within new environments. In agriculture, these measures are aimed at protecting food crops and livestock from pests, invasive species, and other organisms not conducive to the welfare of the human population. The term includes biological threats to people, including those from pandemic diseases and bioterrorism. The definition has sometimes been broadened to embrace other concepts, and it is used for different purposes in different contexts.
A biosafety level (BSL), or pathogen/protection level, is a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels of containment range from the lowest biosafety level 1 (BSL-1) to the highest at level 4 (BSL-4). In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels in a publication referred to as BMBL. In the European Union, the same biosafety levels are defined in a directive. In Canada the four levels are known as Containment Levels. Facilities with these designations are also sometimes given as P1 through P4, as in the term P3 laboratory.

A biological hazard, or biohazard, is a biological substance that poses a threat to the health of living organisms, primarily humans. This could include a sample of a microorganism, virus or toxin that can adversely affect human health. A biohazard could also be a substance harmful to other living beings.
The National Microbiology Laboratory (NML) is part of the Public Health Agency of Canada (PHAC), the agency of the Government of Canada that is responsible for public health, health emergency preparedness and response, and infectious and chronic disease control and prevention.

The United States Army Medical Research Institute of Infectious Diseases is the United States Army's main institution and facility for defensive research into countermeasures against biological warfare. It is located on Fort Detrick, Maryland, near Washington, D.C., and is a subordinate lab of the United States Army Medical Research and Development Command (USAMRDC), headquartered on the same installation.
Infection prevention and control is the discipline concerned with preventing healthcare-associated infections; a practical rather than academic sub-discipline of epidemiology. In Northern Europe, infection prevention and control is expanded from healthcare into a component in public health, known as "infection protection". It is an essential part of the infrastructure of health care. Infection control and hospital epidemiology are akin to public health practice, practiced within the confines of a particular health-care delivery system rather than directed at society as a whole.
Biorisk generally refers to the risk associated with biological materials and/or infectious agents, also known as pathogens. The term has been used frequently for various purposes since the early 1990s. The term is used by regulators, security experts, laboratory personnel and industry alike, and is used by the World Health Organization (WHO). WHO/Europe also provides tools and training courses in biosafety and biosecurity.

In health care facilities, isolation represents one of several measures that can be taken to implement in infection control: the prevention of communicable diseases from being transmitted from a patient to other patients, health care workers, and visitors, or from outsiders to a particular patient. Various forms of isolation exist, in some of which contact procedures are modified, and others in which the patient is kept away from all other people. In a system devised, and periodically revised, by the U.S. Centers for Disease Control and Prevention (CDC), various levels of patient isolation comprise application of one or more formally described "precaution".
The Canadian Science Centre for Human and Animal Health (CSCHAH) is an infectious disease laboratory complex in Winnipeg, Manitoba, owned and operated by the Government of Canada. This modern facility is home to two laboratories: the Public Health Agency of Canada's National Microbiology Laboratory (NML) and the Canadian Food Inspection Agency's National Centre for Foreign Animal Disease (NCFAD). It was the workplace of approximately 550 federal employees prior to the Covid-19 outbreak; since then it has been home to over 800 staff.

A biosafety cabinet (BSC)—also called a biological safety cabinet or microbiological safety cabinet—is an enclosed, ventilated laboratory workspace for safely working with materials contaminated with pathogens requiring a defined biosafety level. Several different types of BSC exist, differentiated by the degree of biocontainment they provide. BSCs first became commercially available in 1950.
Ravn virus is a close relative of Marburg virus (MARV). RAVV causes Marburg virus disease in humans and nonhuman primates, a form of viral hemorrhagic fever. RAVV is a Select agent, World Health Organization Risk Group 4 Pathogen, National Institutes of Health/National Institute of Allergy and Infectious Diseases Category A Priority Pathogen, Centers for Disease Control and Prevention Category A Bioterrorism Agent, and listed as a Biological Agent for Export Control by the Australia Group.

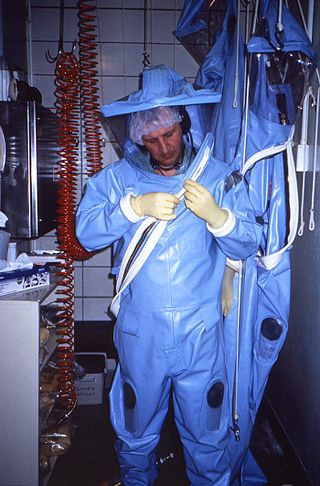
Positive pressure personnel suits (PPPS)—or positive pressure protective suits, informally known as "space suits", "moon suits", "blue suits", etc.—are highly specialized, totally encapsulating, industrial protection inflatable garments worn only within special biocontainment or maximum containment (BSL-4) laboratory facilities. These facilities research dangerous pathogens which are highly infectious and may have no treatments or vaccines available. These facilities also feature other special equipment and procedures such as airlock entry, quick-drench disinfectant showers, special waste disposal systems, and shower exits.
Biosecurity in the United States is governed by the Bureau of Western Hemisphere Affairs, which is part of the US Department of State. It obtains guidance and advice on specific matters relating to biosecurity from various other government agencies.

The Aeromedical Isolation Team of the US Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick, Maryland was a military rapid response team with worldwide airlift capability designed to safely evacuate and manage contagious patients under high-level (BSL-4) bio-containment conditions. Created in 1978, during its final years the AIT was one of MEDCOM’s Special Medical Augmentation Response Teams comprising a portable containment laboratory along with its transit isolators for patient transport. Contingency missions included bioterrorism scenarios as well as the extraction of scientists with exotic infections from remote sites in foreign countries. The AIT trained continuously and was often put on alert status, but only deployed for “real world” missions four times. The AIT was decommissioned in 2010 and its mission was assumed by one of the US Air Force’s Critical Care Air Transport Teams (CCATTs).
The United States Biological Defense Program—in recent years also called the National Biodefense Strategy—refers to the collective effort by all levels of government, along with private enterprise and other stakeholders, in the United States to carry out biodefense activities.
The American Biological Safety Association (ABSA) was founded in 1984 to promote biological safety as an essential principle and serve the needs of biosafety professionals. The association's goals are to represent the interests and desires of practitioners of biosafety, and to be a dispenser of biosafety information.
The hazards of synthetic biology include biosafety hazards to workers and the public, biosecurity hazards stemming from deliberate engineering of organisms to cause harm, and hazards to the environment. The biosafety hazards are similar to those for existing fields of biotechnology, mainly exposure to pathogens and toxic chemicals; however, novel synthetic organisms may have novel risks. For biosecurity, there is concern that synthetic or redesigned organisms could theoretically be used for bioterrorism. Potential biosecurity risks include recreating known pathogens from scratch, engineering existing pathogens to be more dangerous, and engineering microbes to produce harmful biochemicals. Lastly, environmental hazards include adverse effects on biodiversity and ecosystem services, including potential changes to land use resulting from agricultural use of synthetic organisms.
Pandemic prevention is the organization and management of preventive measures against pandemics. Those include measures to reduce causes of new infectious diseases and measures to prevent outbreaks and epidemics from becoming pandemics.
A laboratory-acquired infection or LAI is an infection that is acquired in a laboratory, usually as part of a medical research facility or hospital.








