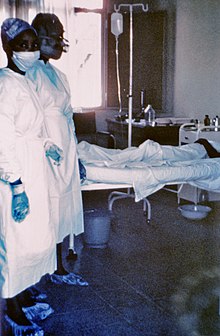
Lassa fever, also known as Lassa hemorrhagic fever, is a type of viral hemorrhagic fever caused by the Lassa virus. Many of those infected by the virus do not develop symptoms. When symptoms occur they typically include fever, weakness, headaches, vomiting, and muscle pains. Less commonly there may be bleeding from the mouth or gastrointestinal tract. The risk of death once infected is about one percent and frequently occurs within two weeks of the onset of symptoms. Of those who survive, about a quarter have hearing loss, which improves within three months in about half of these cases.
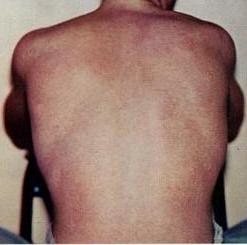
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. Symptoms typically begin 3 to 14 days after infection. These may include a high fever, headache, vomiting, muscle and joint pains, and a characteristic skin itching and skin rash. Recovery generally takes two to seven days. In a small proportion of cases, the disease develops into a more severe dengue hemorrhagic fever, resulting in bleeding, low levels of blood platelets and blood plasma leakage, or into dengue shock syndrome, where dangerously low blood pressure occurs.

Marburg virus disease is a viral hemorrhagic fever in human and non-human primates caused by either of the two Marburgviruses: Marburg virus (MARV) and Ravn virus (RAVV). Its clinical symptoms are very similar to those of Ebola virus disease (EVD).

Ribavirin, also known as tribavirin, is an antiviral medication used to treat RSV infection, hepatitis C and some viral hemorrhagic fevers. For hepatitis C, it is used in combination with other medications such as simeprevir, sofosbuvir, peginterferon alfa-2b or peginterferon alfa-2a. Among the viral hemorrhagic fevers it is sometimes used for Lassa fever, Crimean–Congo hemorrhagic fever, and Hantavirus infection but should not be used for Ebola or Marburg infections. Ribavirin is taken by mouth or inhaled. Despite widespread usage, since the 2010s it has faced scrutiny for a lack of efficacy in treating viral infections it has historically been prescribed for.
Bolivian hemorrhagic fever (BHF), also known as black typhus or Ordog Fever, is a hemorrhagic fever and zoonotic infectious disease originating in Bolivia after infection by Machupo mammarenavirus.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.

An arenavirus is a bi- or trisegmented ambisense RNA virus that is a member of the family Arenaviridae. These viruses infect rodents and occasionally humans. A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease. At least eight arenaviruses are known to cause human disease. The diseases derived from arenaviruses range in severity. Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus. Hemorrhagic fever syndromes, including Lassa fever, are derived from infections such as Guanarito virus, Junin virus, Lassa virus, Lujo virus, Machupo virus, Sabia virus, or Whitewater Arroyo virus. Because of the epidemiological association with rodents, some arenaviruses and bunyaviruses are designated as roboviruses.
Venezuelan hemorrhagic fever (VHF) is a zoonotic human illness first identified in 1989. The disease is most prevalent in several rural areas of central Venezuela and is caused by Guanarito mammarenavirus (GTOV) which belongs to the Arenaviridae family. The short-tailed cane mouse is the main host for GTOV which is spread mostly by inhalation of aerosolized droplets of saliva, respiratory secretions, urine, or blood from infected rodents. Person-to-person spread is possible, but uncommon.

Crimean–Congo hemorrhagic fever (CCHF) is a viral disease. Symptoms of CCHF may include fever, muscle pains, headache, vomiting, diarrhea, and bleeding into the skin. Onset of symptoms is less than two weeks following exposure. Complications may include liver failure. Survivors generally recover around two weeks after onset.
Clarence James Peters, Jr is a physician, field virologist and former U.S. Army colonel. He is noted for his efforts in trying to stem epidemics of exotic infectious diseases such as the Ebola virus, Hanta virus and Rift Valley fever (RVF). He is an eminent authority on the virology, pathogenesis and epidemiology of hemorrhagic fever viruses.
Lujo is a bisegmented RNA virus—a member of the family Arenaviridae—and a known cause of viral hemorrhagic fever (VHF) in humans. Its name was suggested by the Special Pathogens Unit of the National Institute for Communicable Diseases of the National Health Laboratory Service (NICD-NHLS) by using the first two letters of the names of the cities involved in the 2008 outbreak of the disease, Lusaka (Zambia) and Johannesburg. It is the second pathogenic Arenavirus to be described from the African continent—the first being Lassa virus—and since 2012 has been classed as a "Select Agent" under U.S. law.

Marburg virus (MARV) is a hemorrhagic fever virus of the Filoviridae family of viruses and a member of the species Marburg marburgvirus, genus Marburgvirus. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The virus is considered to be extremely dangerous. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen. In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group.
Hantaan orthohantavirus (HTNV) is an enveloped, single-stranded, negative-sense RNA virus species of Old World Orthohantavirus. It is the causative agent of Korean hemorrhagic fever in humans. It is named for the Hantan River in South Korea, and in turn lends the name to its genus Orthohantavirus and family Hantaviridae.

Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever in humans and other primates, caused by ebolaviruses. Symptoms typically start anywhere between two days and three weeks after infection. The first symptoms are usually fever, sore throat, muscle pain, and headaches. These are usually followed by vomiting, diarrhoea, rash and decreased liver and kidney function, at which point some people begin to bleed both internally and externally. It kills between 25% and 90% of those infected – about 50% on average. Death is often due to shock from fluid loss, and typically occurs between six and 16 days after the first symptoms appear. Early treatment of symptoms increases the survival rate considerably compared to late start. An Ebola vaccine was approved by the US FDA in December 2019.

Zaire ebolavirus, more commonly known as Ebola virus, is one of six known species within the genus Ebolavirus. Four of the six known ebolaviruses, including EBOV, cause a severe and often fatal hemorrhagic fever in humans and other mammals, known as Ebola virus disease (EVD). Ebola virus has caused the majority of human deaths from EVD, and was the cause of the 2013–2016 epidemic in western Africa, which resulted in at least 28,646 suspected cases and 11,323 confirmed deaths.

FGI-106 is a broad-spectrum antiviral drug developed as a potential treatment for enveloped RNA viruses, in particular viral hemorrhagic fevers from the bunyavirus, flavivirus and filovirus families. It acts as an inhibitor which blocks viral entry into host cells. In animal tests FGI-106 shows both prophylactic and curative action against a range of deadly viruses for which few existing treatments are available, including the bunyaviruses hantavirus, Rift Valley fever virus and Crimean-Congo hemorrhagic fever virus, the flavivirus dengue virus, and the filoviruses Ebola virus and Marburg virus.
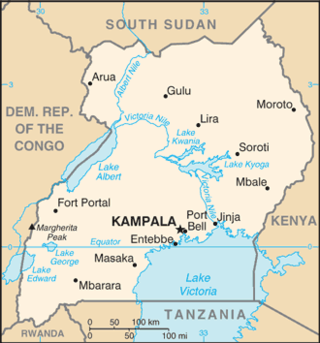
The 2017 Uganda Marburg virus outbreak was confirmed by the World Health Organization (WHO) on 20 October 2017 after there had been an initial fatality due to the virus.

The 1967 Marburg virus outbreak was the first recorded outbreak of Marburg virus disease. It started in early August 1967 when 30 people became ill in the West German towns of Marburg and Frankfurt and later two in Belgrade, Yugoslavia. The infections were traced back to three laboratories in the separate locations which received a shared shipment of infected African green monkeys. The outbreak involved 25 primary Marburg virus infections and seven deaths, and six non-lethal secondary cases.

Orthornavirae is a kingdom of viruses that have genomes made of ribonucleic acid (RNA), including genes which encode an RNA-dependent RNA polymerase (RdRp). The RdRp is used to transcribe the viral RNA genome into messenger RNA (mRNA) and to replicate the genome. Viruses in this kingdom share a number of characteristics which promote rapid evolution, including high rates of genetic mutation, recombination, and reassortment.