Lassa fever, also known as Lassa hemorrhagic fever, is a type of viral hemorrhagic fever caused by the Lassa virus. Many of those infected by the virus do not develop symptoms. When symptoms occur they typically include fever, weakness, headaches, vomiting, and muscle pains. Less commonly there may be bleeding from the mouth or gastrointestinal tract. The risk of death once infected is about one percent and frequently occurs within two weeks of the onset of symptoms. Of those who survive, about a quarter have hearing loss, which improves within three months in about half of these cases.
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. It is frequently asymptomatic; if symptoms appear they typically begin 3 to 14 days after infection. These may include a high fever, headache, vomiting, muscle and joint pains, and a characteristic skin itching and skin rash. Recovery generally takes two to seven days. In a small proportion of cases, the disease develops into a more severe dengue hemorrhagic fever, resulting in bleeding, low levels of blood platelets and blood plasma leakage, or into dengue shock syndrome, where dangerously low blood pressure occurs.

Chikungunya is an infection caused by the Chikungunya virus (CHIKV). The disease was first identified in 1952 in Tanzania and named based on the Kimakonde words for "to become contorted". Symptoms include fever and joint pain. These typically occur two to twelve days after exposure. Other symptoms may include headache, muscle pain, joint swelling, and a rash. Symptoms usually improve within a week; however, occasionally the joint pain may last for months or years. The risk of death is around 1 in 1,000. The very young, old, and those with other health problems are at risk of more severe disease.
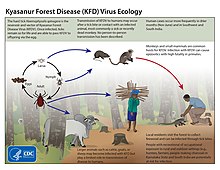
Tick-borne diseases, which afflict humans and other animals, are caused by infectious agents transmitted by tick bites. They are caused by infection with a variety of pathogens, including rickettsia and other types of bacteria, viruses, and protozoa. The economic impact of tick-borne diseases is considered to be substantial in humans, and tick-borne diseases are estimated to affect ~80 % of cattle worldwide. Most of these pathogens require passage through vertebrate hosts as part of their life cycle. Tick-borne infections in humans, farm animals, and companion animals are primarily associated with wildlife animal reservoirs. many tick-borne infections in humans involve a complex cycle between wildlife animal reservoirs and tick vectors. The survival and transmission of these tick-borne viruses are closely linked to their interactions with tick vectors and host cells. These viruses are classified into different families, including Asfarviridae, Reoviridae, Rhabdoviridae, Orthomyxoviridae, Bunyaviridae, and Flaviviridae.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.

Viral hemorrhagic fevers (VHFs) are a diverse group of animal and human illnesses. VHFs may be caused by five distinct families of RNA viruses: the families Filoviridae, Flaviviridae, Rhabdoviridae, and several member families of the Bunyavirales order such as Arenaviridae, and Hantaviridae. All types of VHF are characterized by fever and bleeding disorders and all can progress to high fever, shock and death in many cases. Some of the VHF agents cause relatively mild illnesses, such as the Scandinavian nephropathia epidemica, while others, such as Ebola virus, can cause severe, life-threatening disease.
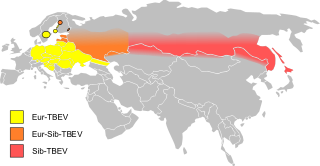
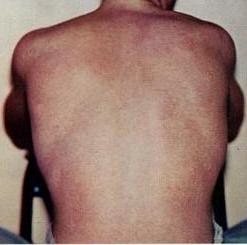
Tick-borne encephalitis (TBE) is a viral infectious disease involving the central nervous system. The disease most often manifests as meningitis, encephalitis or meningoencephalitis. Myelitis and spinal paralysis also occurs. In about one third of cases sequelae, predominantly cognitive dysfunction, persist for a year or more.

Crimean–Congo hemorrhagic fever (CCHF) is a viral disease. Symptoms of CCHF may include fever, muscle pains, headache, vomiting, diarrhea, and bleeding into the skin. Onset of symptoms is less than two weeks following exposure. Complications may include liver failure. Survivors generally recover around two weeks after onset.

Thogotovirus is a genus of enveloped RNA viruses, one of seven genera in the virus family Orthomyxoviridae. Their single-stranded, negative-sense RNA genome has six or seven segments. Thogotoviruses are distinguished from most other orthomyxoviruses by being arboviruses – viruses that are transmitted by arthropods, in this case usually ticks. Thogotoviruses can replicate in both tick cells and vertebrate cells; one subtype has also been isolated from mosquitoes. A consequence of being transmitted by blood-sucking vectors is that the virus must spread systemically in the vertebrate host – unlike influenza viruses, which are transmitted by respiratory droplets and are usually confined to the respiratory system.
Alkhurma virus (ALKV) is a zoonotic virus of the Flaviviridae virus family. ALKV causes Alkhurma hemorrhagic fever (AHF), or alternatively termed as Alkhurma hemorrhagic fever virus, and is mainly based in Saudi Arabia.
Omsk hemorrhagic fever is a viral hemorrhagic fever caused by a Flavivirus.
Mayaro virus disease is a mosquito-borne zoonotic pathogen endemic to certain humid forests of tropical South America. Infection with Mayaro virus causes an acute, self-limited dengue-like illness of 3–5 days' duration. The causative virus, abbreviated MAYV, is in the family Togaviridae, and genus Alphavirus. It is closely related to other alphaviruses that produce a dengue-like illness accompanied by long-lasting arthralgia. It is only known to circulate in tropical South America.
Langat virus (LGTV) is a virus of the genus Flavivirus. The virus was first isolated in Malaysia in 1956 from a hard tick of the Ixodes genus. This virus is antigenically related to Omsk hemorrhagic fever virus, Kyasanur forest disease virus, Alkhurma virus, Louping ill virus and other viruses of the tick-borne encephalitis virus (TBEV) complex. The Langat virus does not pose a significant epidemiological threat in comparison with TBEV. There are no known cases of human diseases associated with LGTV. The Malaysian strain is naturally attenuated and induces neutralizing antibodies to tick-borne encephalitis virus (TBEV) and protection against other TBEV complex viruses in animals.

Marburg virus (MARV) is a hemorrhagic fever virus of the Filoviridae family of viruses and a member of the species Marburg marburgvirus, genus Marburgvirus. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The virus is considered to be extremely dangerous. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen. In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group.
In 1954 the Hazara orthonairovirus, one of the 34 tick-borne viruses of the genus Orthonairovirus, was discovered in Pakistan in the Ixodes tick native to that region. Today this virus is studied in mice in an attempt to develop treatments for the highly pathogenic Crimean-Congo Hemorrhagic Fever virus.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
Pylore Krishnaier Rajagopalan is an Indian vector control scientist, biologist and acarologist, known for his pioneering contributions to the control programmes against vector-borne diseases in India. He is a former director of the Indian Council of Medical Research managed Vector Control Research Centre, Pondicherry. He graduated in 1949 from the Banaras Hindu University and obtained a Masters in Zoology with University First Rank there itself in 1951. In 1952 he joined the fledgling Virus Research Centre in Pune, and worked under the supervision of some of the finest vector control specialists such as Dr T Ramachandra Rao. In recognition of his outstanding work as a young research scientist, in 1957 he was awarded a Fellowship by the Rockefeller Foundation to pursue a Master's program in Public Health from the University of California. He went on to secure a Diploma in Acarology from the University of Maryland at College Park.

Ornithodoros savignyi, known as sand tampan, African eyed tampan or Kalahari sand tampan, is one of some 37 species in the genus Ornithodoros and is a soft tick with a leathery, mammillated integument, causing paralysis and tampan toxicosis, two unrelated conditions. The sand tampan is an ectoparasite on humans, their livestock and wild animals, including birds and bats. Occurring in semi-desert areas of Africa, Saudi Arabia and other parts of the Persian Gulf, India, Sri Lanka and into Asia, it is able to survive for lengthy periods without feeding, spending most of its life burrowed under sand or loose soil, often in wait for animals that rest or sleep under trees or in the lee of rocks, but also in places where people or their animals congregate such as marketplaces, places of worship, cattle kraals and village squares. The timing of its activity is geared to coincide with that of potential hosts, but hot sunny conditions are usually avoided. Because of its habit of feeding and dropping from its host, adult dispersal is limited, whereas larvae may remain attached to their hosts for several days. During its life cycle it will feed on multiple hosts between moults.

MK-608 is an antiviral drug, an adenosine analog. It was originally developed by Merck & Co. as a treatment for hepatitis C, but despite promising results in animal studies, it was ultimately unsuccessful in clinical trials. Subsequently it has been widely used in antiviral research and has shown activity against a range of viruses, including Dengue fever, tick-borne encephalitis virus, poliovirus, and most recently Zika virus, in both in vitro and animal models. Since it has already failed in human clinical trials previously, it is unlikely MK-608 itself will be developed as an antiviral medication, but the continuing lack of treatment options for these emerging viral diseases means that much research continues using MK-608 and related antiviral drugs.
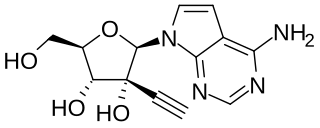
NITD008 is an antiviral drug classified as an adenosine analog. It was developed as a potential treatment for flavivirus infections and shows broad spectrum antiviral activity against many related viruses such as dengue virus, West Nile virus, yellow fever virus, Powassan virus, hepatitis C virus, Kyasanur Forest disease virus, Omsk hemorrhagic fever virus, and Zika virus. However, NITD008 proved too toxic in pre-clinical animal testing to be suitable for human trials, but it continues to be used in research to find improved treatments for emerging viral diseases.