| Sindbis virus | |
|---|---|
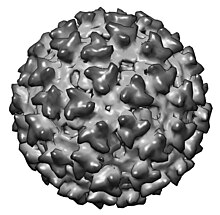 | |
| CryoEM model of Sindbis virus. EMDB entry EMD-2374 [1] | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Orthornavirae |
| Phylum: | Kitrinoviricota |
| Class: | Alsuviricetes |
| Order: | Martellivirales |
| Family: | Togaviridae |
| Genus: | Alphavirus |
| Species: | Sindbis virus |
Sindbis virus (SINV) is a member of the Togaviridae family, in the Alphavirus genus. The virus was first isolated in 1952 in Cairo, Egypt. [2] The virus is transmitted by mosquitoes ( Culex and Culiseta). SINV is linked to Pogosta disease [3] (Finland), Ockelbo disease (Sweden) and Karelian fever (Russia). In humans, the symptoms include arthralgia, rash and malaise. Sindbis virus is widely and continuously found in insects and vertebrates in Eurasia, Africa, and Oceania. Clinical infection and disease in humans however has almost only been reported from Northern Europe (Finland, Sweden, Russian Karelia), where SINV is endemic and where large outbreaks occur intermittently. Cases are occasionally reported in Australia, China, and South Africa. [4]
Contents
SINV is an arbovirus, it is arthropod-borne, and it is maintained in nature by transmission between vertebrate (bird) hosts and invertebrate (mosquito) vectors. Humans are infected with Sindbis virus when bitten by an infected mosquito.