
CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as helper T cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.
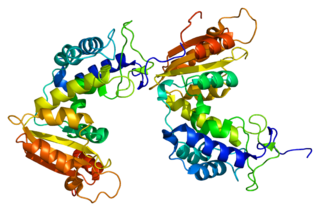
CD38 (cluster of differentiation 38), also known as cyclic ADP ribose hydrolase is a glycoprotein found on the surface of many immune cells (white blood cells), including CD4+, CD8+, B lymphocytes and natural killer cells. CD38 also functions in cell adhesion, signal transduction and calcium signaling.

The chemokine ligand 2 (CCL2) is also referred to as monocyte chemoattractant protein 1 (MCP1) and small inducible cytokine A2. CCL2 is a small cytokine that belongs to the CC chemokine family. CCL2 tightly regulates cellular mechanics and thereby recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.

Integrin alpha M (ITGAM) is one protein subunit that forms heterodimeric integrin alpha-M beta-2 (αMβ2) molecule, also known as macrophage-1 antigen (Mac-1) or complement receptor 3 (CR3). ITGAM is also known as CR3A, and cluster of differentiation molecule 11B (CD11B). The second chain of αMβ2 is the common integrin β2 subunit known as CD18, and integrin αMβ2 thus belongs to the β2 subfamily integrins.

Thy-1 or CD90 is a 25–37 kDa heavily N-glycosylated, glycophosphatidylinositol (GPI) anchored conserved cell surface protein with a single V-like immunoglobulin domain, originally discovered as a thymocyte antigen. Thy-1 can be used as a marker for a variety of stem cells and for the axonal processes of mature neurons. Structural study of Thy-1 led to the foundation of the Immunoglobulin superfamily, of which it is the smallest member, and led to some of the initial biochemical description and characterization of a vertebrate GPI anchor and also the first demonstration of tissue specific differential glycosylation.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

EGF-like module-containing mucin-like hormone receptor-like 1 also known as F4/80 is a protein encoded by the ADGRE1 gene. EMR1 is a member of the adhesion GPCR family. Adhesion GPCRs are characterized by an extended extracellular region often possessing N-terminal protein modules that is linked to a TM7 region via a domain known as the GPCR-Autoproteolysis INducing (GAIN) domain.

CD163 is a protein that in humans is encoded by the CD163 gene. CD163 is the high affinity scavenger receptor for the hemoglobin-haptoglobin complex and in the absence of haptoglobin - with lower affinity - for hemoglobin alone. It also is a marker of cells from the monocyte/macrophage lineage. CD163 functions as innate immune sensor for gram-positive and gram-negative bacteria. The receptor was discovered in 1987.

4F2 cell-surface antigen heavy chain is a protein that in humans is encoded by the SLC3A2 gene.

Colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115, is a cell-surface protein encoded by the human CSF1R gene. CSF1R is a receptor that can be activated by two ligands: colony stimulating factor 1 (CSF-1) and interleukin-34 (IL-34). CSF1R is highly expressed in myeloid cells, and CSF1R signaling is necessary for the survival, proliferation, and differentiation of many myeloid cell types in vivo and in vitro. CSF1R signaling is involved in many diseases and is targeted in therapies for cancer, neurodegeneration, and inflammatory bone diseases.

CD48 antigen also known as B-lymphocyte activation marker (BLAST-1) or signaling lymphocytic activation molecule 2 (SLAMF2) is a protein that in humans is encoded by the CD48 gene.

OX-2 membrane glycoprotein, also named CD200 is a human protein encoded by the CD200 gene. CD200 gene is in human located on chromosome 3 in proximity to genes encoding other B7 proteins CD80/CD86. In mice CD200 gene is on chromosome 16.

Macrophage receptor with collagenous structure (MARCO) is a protein that in humans is encoded by the MARCO gene. MARCO is a class A scavenger receptor that is found on particular subsets of macrophages. Scavenger receptors are pattern recognition receptors (PRRs) found most commonly on immune cells. Their defining feature is that they bind to polyanions and modified forms of a type of cholesterol called low-density lipoprotein (LDL). MARCO is able to bind and phagocytose these ligands and pathogen-associated molecular patterns (PAMPs), leading to the clearance of pathogens and cell signaling events that lead to inflammation. As part of the innate immune system, MARCO clears, or scavenges, pathogens, which leads to inflammatory responses. The scavenger receptor cysteine-rich (SRCR) domain at the end of the extracellular side of MARCO binds ligands to activate the subsequent immune responses. MARCO expression on macrophages has been associated with tumor development and also with Alzheimer's disease, via decreased responses of cells when ligands bind to MARCO.

Lysosome-associated membrane glycoprotein 3 is a protein that in humans is encoded by the LAMP3 gene. It is one of the lysosome-associated membrane glycoproteins.
The following outline is provided as an overview of and topical guide to immunology:
Lysosome-associated membrane glycoproteins (LAMPs) are integral membrane proteins, specific to lysosomes, and whose exact biological function is not yet clear. Structurally, the lamp proteins consist of two internally homologous lysosome-luminal domains separated by a proline-rich hinge region; at the C-terminal extremity there is a transmembrane region (TM) followed by a very short cytoplasmic tail (C). In each of the duplicated domains, there are two conserved disulfide bonds. This structure is schematically represented in the figure below.
+-----+ +-----+ +-----+ +-----+ | | | | | | | | xCxxxxxCxxxxxxxxxxxxCxxxxxCxxxxxxxxxCxxxxxCxxxxxxxxxxxxCxxxxxCxxxxxxxx +--------------------------++Hinge++--------------------------++TM++C+
Liver sinusoidal endothelial cells (LSECs) form the lining of the smallest blood vessels in the liver, also called the hepatic sinusoids. LSECs are highly specialized endothelial cells with characteristic morphology and function. They constitute an important part of the reticuloendothelial system (RES).



















