Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer.

The selectins are a family of cell adhesion molecules. All selectins are single-chain transmembrane glycoproteins that share similar properties to C-type lectins due to a related amino terminus and calcium-dependent binding. Selectins bind to sugar moieties and so are considered to be a type of lectin, cell adhesion proteins that bind sugar polymers.
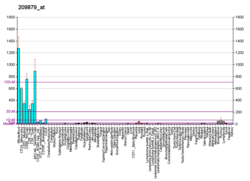
The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene on chromosome 11. CD44 has been referred to as HCAM, Pgp-1, Hermes antigen, lymphocyte homing receptor, ECM-III, and HUTCH-1.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.

Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the PTPN11 gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2.
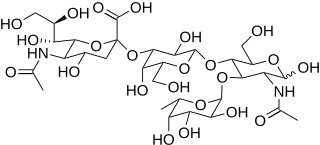
Sialyl LewisX (sLeX), also known as cluster of differentiation 15s (CD15s) or stage-specific embryonic antigen 1 (SSEA-1), is a tetrasaccharide carbohydrate which is usually attached to O-glycans on the surface of cells. It is known to play a vital role in cell-to-cell recognition processes. It is also the means by which an egg attracts sperm; first, to stick to it, then bond with it and eventually form a zygote.

Integrin beta-3 (β3) or CD61 is a protein that in humans is encoded by the ITGB3 gene. CD61 is a cluster of differentiation found on thrombocytes.

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Signal regulatory protein α (SIRPα) is a regulatory membrane glycoprotein from SIRP family expressed mainly by myeloid cells and also by stem cells or neurons.

Glycoprotein Ib (platelet), beta polypeptide (GP1BB) also known as CD42c, is a protein that in humans is encoded by the GP1BB gene.

CD166 antigen is a 100-105 kD typeI transmembrane glycoprotein that is a member of the immunoglobulin superfamily of proteins. In humans it is encoded by the ALCAM gene. It is also called CD166, MEMD, SC-1/DM-GRASP/BEN in the chicken, and KG-CAM in the rat.

Beta-1,3-galactosyl-O-glycosyl-glycoprotein beta-1,6-N-acetylglucosaminyltransferase is an enzyme that in humans is encoded by the GCNT1 gene.

Glycoprotein V (platelet) (GP5) also known as CD42d (Cluster of Differentiation 42d), is a human gene.

Carbohydrate sulfotransferase 2 is an enzyme that in humans is encoded by the CHST2 gene.

Carbohydrate sulfotransferase 4 is an enzyme that in humans is encoded by the CHST4 gene.

Carbohydrate sulfotransferase 1 is an enzyme that in humans is encoded by the CHST1 gene.

Disintegrin and metalloproteinase domain-containing protein 28 is an enzyme that in humans is encoded by the ADAM28 gene.
A catch bond is a type of noncovalent bond whose dissociation lifetime increases with tensile force applied to the bond. Normally, bond lifetimes are expected to diminish with force. In the case of catch bonds, the lifetime of the bond actually increases up to a maximum before it decreases like in a normal bond. Catch bonds work in a way that is conceptually similar to that of a Chinese finger trap. While catch bonds are strengthened by an increase in force, the force increase is not necessary for the bond to work. Catch bonds were suspected for many years to play a role in the rolling of leukocytes, being strong enough to roll in presence of high forces caused by high shear stresses, while avoiding getting stuck in capillaries where the fluid flow, and therefore shear stress, is low. The existence of catch bonds was debated for many years until strong evidence of their existence was found in bacteria. Definite proof of their existence came shortly thereafter in leukocytes.