Signal transducer CD24 also known as cluster of differentiation 24 or heat stable antigen CD24 (HSA) is a protein that in humans is encoded by the CD24 gene. [5] CD24 is a cell adhesion molecule.
Signal transducer CD24 also known as cluster of differentiation 24 or heat stable antigen CD24 (HSA) is a protein that in humans is encoded by the CD24 gene. [5] CD24 is a cell adhesion molecule.
CD24 is a sialoglycoprotein expressed at the surface of most B lymphocytes and differentiating neuroblasts. It is also expressed on neutrophils [6] and neutrophil precursors from the myelocyte stage onwards. The encoded protein is anchored via a glycosyl phosphatidylinositol (GPI) link to the cell surface. The protein also contributes to a wide range of downstream signaling networks and is crucial for neural development. [7] Cross-linking of CD24 on the surface of neutrophils induces apoptosis, [8] and this appears to be defective in sepsis. [8] CD24 gene is found on chromosome 6 (6q21) An alignment of this gene's sequence finds genomic locations with similarity on chromosomes 1p36, 3p26, 15q21.3, 20q11.2 and Yq11.222. Whether transcription, and corresponding translation, occurs at each of these other genomic locations needs to be experimentally determined.[ citation needed ]
Researchers have identified CD24 as a novel cell surface marker that flags anastasis in melanoma cells, a process where cells that have initiated apoptosis can recover and survive. [9] This discovery highlights CD24’s role in marking apoptotic subpopulations that exhibit metabolic activity and proliferative capacities, contributing to melanoma’s resilience and potential metastasis.
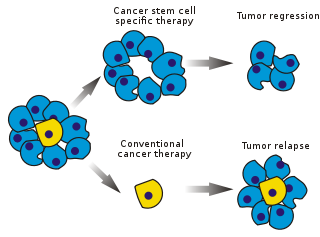
Cancer stem cells (CSCs) are cancer cells that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are hypothesized to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for patients with metastatic disease.

The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene on chromosome 11. CD44 has been referred to as HCAM, Pgp-1, Hermes antigen, lymphocyte homing receptor, ECM-III, and HUTCH-1.

E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.

CD146 also known as the melanoma cell adhesion molecule (MCAM) or cell surface glycoprotein MUC18, is a 113kDa cell adhesion molecule currently used as a marker for endothelial cell lineage. In humans, the CD146 protein is encoded by the MCAM gene.
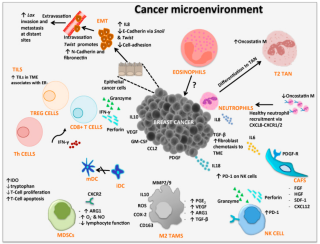
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Mucin short variant S1, also called polymorphic epithelial mucin (PEM) or epithelial membrane antigen (EMA), is a mucin encoded by the MUC1 gene in humans. Mucin short variant S1 is a glycoprotein with extensive O-linked glycosylation of its extracellular domain. Mucins line the apical surface of epithelial cells in the lungs, stomach, intestines, eyes and several other organs. Mucins protect the body from infection by pathogen binding to oligosaccharides in the extracellular domain, preventing the pathogen from reaching the cell surface. Overexpression of MUC1 is often associated with colon, breast, ovarian, lung and pancreatic cancers. Joyce Taylor-Papadimitriou identified and characterised the antigen during her work with breast and ovarian tumors.

Receptor-binding cancer antigen expressed on SiSo cells is a protein that in humans is encoded by the EBAG9 gene.

Cluster of differentiation 97 is a protein also known as BL-Ac[F2] encoded by the ADGRE5 gene. CD97 is a member of the adhesion G protein-coupled receptor (GPCR) family. Adhesion GPCRs are characterized by an extended extracellular region often possessing N-terminal protein modules that is linked to a TM7 region via a domain known as the GPCR-Autoproteolysis INducing (GAIN) domain.

Epithelial cell adhesion molecule (EpCAM), also known as CD326 among other names, is a transmembrane glycoprotein mediating Ca2+-independent homotypic cell–cell adhesion in epithelia. EpCAM is also involved in cell signaling, migration, proliferation, and differentiation. Additionally, EpCAM has oncogenic potential via its capacity to upregulate c-myc, e-fabp, and cyclins A & E. Since EpCAM is expressed exclusively in epithelia and epithelial-derived neoplasms, EpCAM can be used as diagnostic marker for various cancers. It appears to play a role in tumorigenesis and metastasis of carcinomas, so it can also act as a potential prognostic marker and as a potential target for immunotherapeutic strategies.

Serpin B4 is a protein that in humans is encoded by the SERPINB4 gene.

Prostate stem cell antigen is a protein that in humans is encoded by the PSCA gene.

Galectin-8 is a protein of the galectin family that in humans is encoded by the LGALS8 gene.

RNA-binding protein 5 is a protein that in humans is encoded by the RBM5 gene.

Homeobox protein Hox-C6 is a protein that in humans is encoded by the HOXC6 gene. Hox-C6 expression is highest in the fallopian tube and ovary. HoxC6 has been highly expressed in many types of cancers including prostate, breast, and esophageal squamous cell cancer.

Carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3) also known as CD66d, is a member of the carcinoembryonic antigen (CEA) gene family..

CD109 is a human gene.

Epithelial membrane protein 3 (EMP3) is a trans-membrane signaling molecule that is encoded by the myelin-related gene EMP3. EMP3 is a member of the peripheral myelin protein gene family 22-kDa (PMP22), which is mainly responsible for the formation of the sheath of compact myelin. Although the detailed functions and mechanisms of EMP3 still remain unclear, it is suggested that EMP3 is possibly epigenetically linked to certain carcinomas.

Metadherin, also known as protein LYRIC or astrocyte elevated gene-1 protein (AEG-1) is a protein that in humans is encoded by the MTDH gene.

CD205 also called Lymphocyte antigen 75 is a protein that in humans is encoded by the LY75 gene.