| FUT5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | FUT5 , FUC-TV, fucosyltransferase 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 136835 HomoloGene: 134030 GeneCards: FUT5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT5 gene. [3] [4]
| FUT5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | FUT5 , FUC-TV, fucosyltransferase 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 136835 HomoloGene: 134030 GeneCards: FUT5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT5 gene. [3] [4]
A congenital disorder of glycosylation is one of several rare inborn errors of metabolism in which glycosylation of a variety of tissue proteins and/or lipids is deficient or defective. Congenital disorders of glycosylation are sometimes known as CDG syndromes. They often cause serious, sometimes fatal, malfunction of several different organ systems in affected infants. The most common sub-type is PMM2-CDG where the genetic defect leads to the loss of phosphomannomutase 2 (PMM2), the enzyme responsible for the conversion of mannose-6-phosphate into mannose-1-phosphate
A fucosyltransferase is an enzyme that transfers an L-fucose sugar from a GDP-fucose donor substrate to an acceptor substrate. The acceptor substrate can be another sugar such as the transfer of a fucose to a core GlcNAc (N-acetylglucosamine) sugar as in the case of N-linked glycosylation, or to a protein, as in the case of O-linked glycosylation produced by O-fucosyltransferase. There are various fucosyltransferases in mammals, the vast majority of which, are located in the Golgi apparatus. The O-fucosyltransferases have recently been shown to localize to the endoplasmic reticulum (ER).

Beta-hexosaminidase subunit beta is an enzyme that in humans is encoded by the HEXB gene.
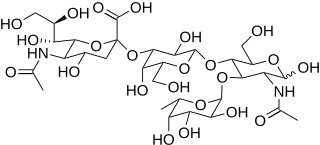
Sialyl LewisX (sLeX), also known as cluster of differentiation 15s (CD15s) or stage-specific embryonic antigen 1 (SSEA-1), is a tetrasaccharide carbohydrate which is usually attached to O-glycans on the surface of cells. It is known to play a vital role in cell-to-cell recognition processes. It is also the means by which an egg attracts sperm; first, to stick to it, then bond with it and eventually form a zygote. The discovery of the essential role that this tetrasaccharide plays in the fertilization process was reported in August 2011.
In enzymology, a glycoprotein 3-alpha-L-fucosyltransferase (EC 2.4.1.214) is an enzyme that catalyzes the chemical reaction

Galactoside 3(4)-L-fucosyltransferase is an enzyme that in humans is encoded by the FUT3 gene.

Galactoside 2-alpha-L-fucosyltransferase 2 is an enzyme that in humans is encoded by the FUT2 gene. It affects the secretor status of ABO antigens.

Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT7 gene.

Galactoside 2-alpha-L-fucosyltransferase 1 is an enzyme that in humans is encoded by the FUT1 gene.

Beta-1,3-galactosyl-O-glycosyl-glycoprotein beta-1,6-N-acetylglucosaminyltransferase is an enzyme that in humans is encoded by the GCNT1 gene.

Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT6 gene.
Alpha-(1,6)-fucosyltransferase is an enzyme that in humans is encoded by the FUT8 gene.

Alpha-1,3/1,6-mannosyltransferase ALG2 is an enzyme that is encoded by the ALG2 gene. Mutations in the human gene are associated with congenital defects in glycosylation The protein encoded by the ALG2 gene belongs to two classes of enzymes: GDP-Man:Man1GlcNAc2-PP-dolichol alpha-1,3-mannosyltransferase and GDP-Man:Man2GlcNAc2-PP-dolichol alpha-1,6-mannosyltransferase.

Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT9 gene.

UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 2 is an enzyme that in humans is encoded by the B3GNT2 gene.

ST3 beta-galactoside alpha-2,3-sialyltransferase 3, also known as ST3GAL3, is a protein which in humans is encoded by the ST3GAL3 gene.

Sialic acid-binding Ig-like lectin 8 is a protein that in humans is encoded by the SIGLEC8 gene. This gene is located on chromosome 19q13.4, about 330 kb downstream of the SIGLEC9 gene. Within the siglec family of transmembrane proteins, Siglec-8 belongs to the CD33-related siglec subfamily, a subfamily that has undergone rapid evolution.
Fucosyltransferase 4 , also known as FUT4, is an enzyme which in humans is encoded by the FUT4 gene.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

Fucosyltransferase 4 is a protein that in humans is encoded by the FUT4 gene.