Nucleotides are organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver.

In biochemistry, a kinase is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donates a phosphate group to the substrate molecule. This transesterification produces a phosphorylated substrate and ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and ADP gains a phosphate group. These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis.

Phosphoglucomutase is an enzyme that transfers a phosphate group on an α-D-glucose monomer from the 1 to the 6 position in the forward direction or the 6 to the 1 position in the reverse direction.
A salvage pathway is a pathway in which a biological product is produced from intermediates in the degradative pathway of its own or a similar substance. The term often refers to nucleotide salvage in particular, in which nucleotides are synthesized from intermediates in their degradative pathway.
A nucleoside triphosphate is a nucleoside containing a nitrogenous base bound to a 5-carbon sugar, with three phosphate groups bound to the sugar. They are the molecular precursors of both DNA and RNA, which are chains of nucleotides made through the processes of DNA replication and transcription. Nucleoside triphosphates also serve as a source of energy for cellular reactions and are involved in signalling pathways.

Adenosine deaminase is an enzyme involved in purine metabolism. It is needed for the breakdown of adenosine from food and for the turnover of nucleic acids in tissues.

Glycogen phosphorylase is one of the phosphorylase enzymes. Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.

5′-Nucleotidase is an enzyme which catalyzes the phosphorylytic cleavage of 5′-nucleotides. Although originally found in snake venom, the activity of 5'nucleotidase has been described for bacteria and plant cells, and is widely distributed in vertebrate tissue. In mammalian cells the enzyme is predominantly located in the plasma membrane and its primary role is in the conversion of extracellular nucleotides, which are generally impermeable, to the corresponding nucleoside which can readily enter most cells. Consequently, the enzyme plays a key role in the metabolism of nucleotides.
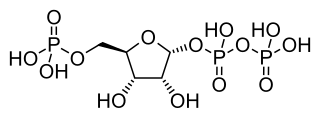
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase:

Nucleic acid metabolism is a collective term that refers to the variety of chemical reactions by which nucleic acids are either synthesized or degraded. Nucleic acids are polymers made up of a variety of monomers called nucleotides. Nucleotide synthesis is an anabolic mechanism generally involving the chemical reaction of phosphate, pentose sugar, and a nitrogenous base. Degradation of nucleic acids is a catabolic reaction and the resulting parts of the nucleotides or nucleobases can be salvaged to recreate new nucleotides. Both synthesis and degradation reactions require multiple enzymes to facilitate the event. Defects or deficiencies in these enzymes can lead to a variety of diseases.

Ribose 5-phosphate (R5P) is both a product and an intermediate of the pentose phosphate pathway. The last step of the oxidative reactions in the pentose phosphate pathway is the production of ribulose 5-phosphate. Depending on the body's state, ribulose 5-phosphate can reversibly isomerize to ribose 5-phosphate. Ribulose 5-phosphate can alternatively undergo a series of isomerizations as well as transaldolations and transketolations that result in the production of other pentose phosphates as well as fructose 6-phosphate and glyceraldehyde 3-phosphate.

Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.

Purine nucleoside phosphorylase deficiency is a rare autosomal recessive metabolic disorder which results in immunodeficiency.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.

Deoxycytidine kinase (dCK) is an enzyme which is encoded by the DCK gene in humans. dCK predominantly phosphorylates deoxycytidine (dC) and converts dC into deoxycytidine monophosphate. dCK catalyzes one of the initial steps in the nucleoside salvage pathway and has the potential to phosphorylate other preformed nucleosides, specifically deoxyadenosine (dA) and deoxyguanosine (dG), and convert them into their monophosphate forms. There has been recent biomedical research interest in investigating dCK's potential as a therapeutic target for different types of cancer.

Thymidine phosphorylase is an enzyme that is encoded by the TYMP gene and catalyzes the reaction:

Adenosine kinase is an enzyme that catalyzes the transfer of gamma-phosphate from Adenosine triphosphate (ATP) to adenosine (Ado) leading to formation of Adenosine monophosphate (AMP). In addition to its well-studied role in controlling the cellular concentration of Ado, AdK also plays an important role in the maintenance of methylation reactions. All S-adenosylmethionine-dependent transmethylation reactions in cells lead to production of S-adenosylhomocysteine (SAH), which is cleaved by SAH hydrolase into Ado and homocysteine. The failure to efficiently remove these end products can result in buildup of SAH, which is a potent inhibitor of all transmethylation reactions. The disruption of AdK gene (-/-) in mice causes neonatal hepatic steatosis, a fatal condition characterized by rapid microvesicular fat infiltration, leading to early postnatal death. The liver was the main organ affected in these animals and in it the levels of adenine nucleotides were decreased, while those of SAH were elevated. Recently, missense mutations in the AdK gene in humans which result in AdK deficiency have also been shown to cause hypermethioninemia, encephalopathy and abnormal liver function.

In enzymology, a ribokinase is an enzyme that catalyzes the chemical reaction
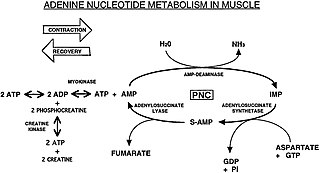
The Purine Nucleotide Cycle is a metabolic pathway in protein metabolism requiring the amino acids aspartate and glutamate. The cycle is used to regulate the levels of adenine nucleotides, in which ammonia and fumarate are generated. AMP coverts into IMP and the byproduct ammonia. IMP converts to S-AMP (adenylosuccinate), which then coverts to AMP and the byproduct fumarate. The fumarate goes on to produce ATP (energy) via oxidative phosphorylation as it enters the Krebs cycle and then the electron transport chain. Lowenstein first described this pathway and outlined its importance in processes including amino acid catabolism and regulation of flux through glycolysis and the Krebs cycle.
Xanthosine phosphorylase, also known as inosine-guanosine phosphorylase, is a catalytic enzyme encoded by the XapA gene in E. coli. The presence of xanthosine is known to induce the synthesis of xanthosine phosphorylase by the XapA gene. The enzyme's main functions are nucleoside phosphorolysis and the synthesis of nucleotides, making it a member of the purine nucleoside phosphorylase group. This protein can degrade all purine nucleosides except adenosine, deoxyadenosine, hypoxanthine arabinoside. These degradation reactions are reversible in vitro, however, phosphorolysis dominates in vivo. Xanthosine phosphorylase is localized in the cytoplasm because these degradation functions take place there. Xanthosine phosphorylase preferentially uses the neutral form of xanthosine over its monoanionic form because it prefers to be in a neutral environment.