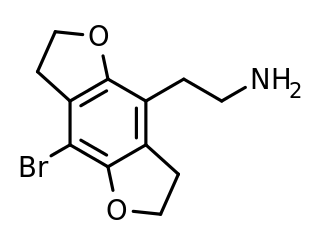
2C-B-FLY is a psychedelic phenethylamine and designer drug of the 2C family. It was first synthesized in 1996 by Aaron Monte, Professor of Chemistry at UW-La Crosse.

Ohmefentanyl is an extremely potent opioid analgesic drug which selectively binds to the μ-opioid receptor.

3-Methylfentanyl is an opioid analgesic that is an analog of fentanyl. 3-Methylfentanyl is one of the most potent opioids, estimated to be between 400 and 6000 times stronger than morphine, depending on which isomer is used.

Lofentanil or lofentanyl is one of the most potent opioid analgesics known and is an analogue of fentanyl, which was developed in 1960. It is most similar to the highly potent opioid carfentanil (4-carbomethoxyfentanyl), only slightly more potent. Lofentanil can be described as 3-methylcarfentanil, or 3-methyl-4-carbomethoxyfentanyl. While 3-methylfentanyl is considerably more potent than fentanyl itself, lofentanil is only slightly stronger than carfentanil. This suggests that substitution at both the 3 and 4 positions of the piperidine ring introduces steric hindrance which prevents μ-opioid affinity from increasing much further. As with other 3-substituted fentanyl derivatives such as ohmefentanyl, the stereoisomerism of lofentanil is very important, with some stereoisomers being much more potent than others.
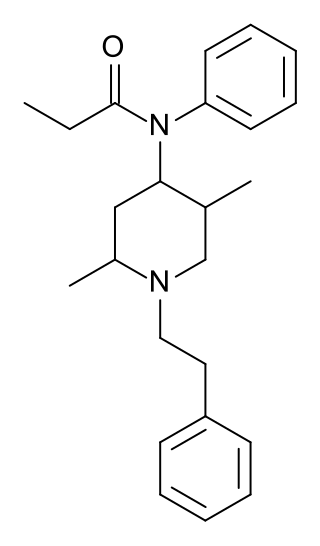
Phenaridine (2,5-dimethylfentanyl) is an opioid analgesic that is an analogue of fentanyl. It was developed in 1972, and is used for surgical anasthesia.

14-Phenylpropoxymetopon (PPOM) is an opioid analogue that is a derivative of metopon which has been substituted with a γ-phenylpropoxy group at the 14-position. PPOM is a highly potent analgesic drug several thousand times stronger than morphine, with an even higher in vivo potency than etorphine. The 14-phenylpropoxy substitution appears to confer potent μ-opioid agonist activity, even when combined with substitutions such as N-cyclopropyl or N-allyl, which normally result in μ-opioid antagonist compounds.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

4-Phenylfentanyl is an opioid analgesic that is a derivative of fentanyl. It was developed during the course of research that ultimately resulted in super-potent opioid derivatives such as carfentanil, though it is a substantially less potent analogue. 4-Phenylfentanyl is around eight times the potency of fentanyl in analgesic tests on animals, but more complex 4-heteroaryl derivatives such as substituted thiophenes and thiazoles are more potent still, as they are closer bioisosteres to the 4-carbomethoxy group of carfentanil.

2CB-Ind is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, discovered in 1974 by Alexander Shulgin. It acts as a moderately potent and selective agonist for the 5-HT2A and 5-HT2C receptors, but unlike the corresponding benzocyclobutene derivative TCB-2 which is considerably more potent than the parent compound 2C-B, 2CB-Ind is several times weaker, with racemic 2CB-Ind having a Ki of 47nM at the human 5-HT2A receptor, only slightly more potent than the mescaline analogue (R)-jimscaline.

R-30490 is an opioid analgesic related to the highly potent animal tranquilizer carfentanil, and with only slightly lower potency. It was first synthesised by a team of chemists at Janssen Pharmaceutica led by Paul Janssen, who were investigating the structure-activity relationships of the fentanyl family of drugs. R-30490 was found to be the most selective agonist for the μ-opioid receptor out of all the fentanyl analogues tested, but it has never been introduced for medical use in humans, although the closely related drug sufentanil is widely used for analgesia and anesthesia during major surgery.

Butyrfentanyl or butyrylfentanyl is a potent short-acting synthetic opioid analgesic drug. It is an analog of fentanyl with around one quarter of its potency. One of the first mentions of this drug can be found in document written by The College on Problem of Drug Dependence, where it is mentioned as N-butyramide fentanyl analog. This document also states that the article describing its clinical effects was published in 1987. It is an agonist for the μ-opioid receptors.

3-Methylbutyrfentanyl (3-MBF) is an opioid analgesic that is an analog of butyrfentanyl.
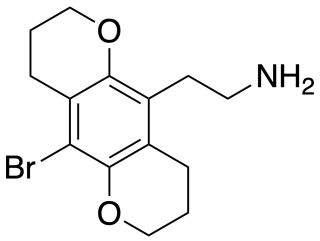
2C-B-BUTTERFLY is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, which was discovered in 1999 by Michael S. Whiteside and Aaron Monte. It is a ring-expanded homologue of the better known compound 2C-B-FLY, and has similar properties as an agonist for serotonin receptors, but with more selectivity for 5-HT2C over 5-HT2A.
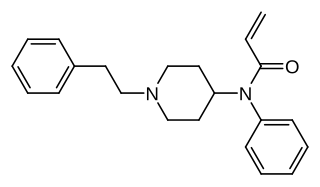
Acrylfentanyl (also known as acryloylfentanyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 (the half maximal inhibitory concentration for acrylfentanyl to displace naloxone) is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.

Isofentanyl (3-methyl-benzylfentanyl) is an opioid analgesic that is an analog of fentanyl first invented in 1973, and which has been sold as a designer drug.

Diphenpipenol is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative related to compounds such as MT-45 and AD-1211, but diphenpipenol is the most potent compound in the series, with the more active (S) enantiomer being around 105 times the potency of morphine in animal studies. This makes it a similar strength to fentanyl and its analogues, and consequently diphenpipenol can be expected to pose a significant risk of producing life-threatening respiratory depression, as well as other typical opioid side effects such as sedation, itching, nausea and vomiting.
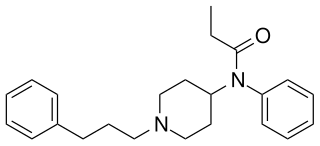
Homofentanyl is an opioid derivative which has been sold as a designer drug. It is a homologue of fentanyl, with similar analgesic and sedative effects but lower potency, around 14× stronger than pethidine.

Fentanyl tropane (tropafentanyl) is an opioid derivative which is an analogue of fentanyl, where the central piperidine ring has been bridged with a two carbon chain to form a tropane ring. It is made from tropinone in a similar manner to how fentanyl is made from 4-piperidone, and has two isomers, with the 3β isomer being around half the potency of fentanyl while the 3α isomer is much weaker, only around the same potency as morphine.


















