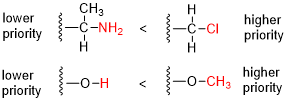
The Cahn–Ingold–Prelog (CIP) sequence rules, named for organic chemists Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog — alternatively termed the CIP priority rules, system, or conventions — are a standard process used in organic chemistry to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of its stereogenic centers will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic and organometallic molecule with all atoms of ligancy of fewer than 4.

Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis-trans notation does not always correspond to E–Z isomerism, which is an absolute stereochemical description. In general, cis–trans stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circular birefringence and circular dichroism are the manifestations of optical activity. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes or metamaterials.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are mirror images of each other that cannot appear identical simply by reorientation. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic form. Half of the optically active substance becomes its mirror image (enantiomer) referred as racemic mixtures. If the racemization results in a mixture where the D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects.

In stereochemistry, a stereogenic element of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, having at least two different groups bound, interchanging any two different groups would create a stereoisomer.

A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereogenic centers, the molecule is not chiral. A meso compound is "superposable" on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite. Both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used.

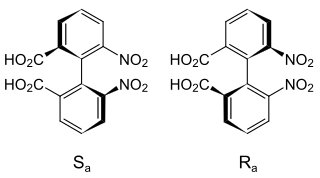
Axial chirality is a special case of chirality in which a molecule does not possess a chiral center but an axis of chirality, an axis about which a set of substituents is held in a spatial arrangement that is not superposable on its mirror image. Axial chirality is most commonly observed in atropisomeric substituted biaryl compounds wherein the rotation about the aryl–aryl bond is restricted, for example, various biphenyls, binaphthyls such as BINAP, and certain dihydroanthracenone compounds. Certain allene compounds and spirans also display axial chirality. The enantiomers of axially chiral compounds are usually given the stereochemical labels Ra and Sa. The designations are based on the same Cahn–Ingold–Prelog priority rules used for tetrahedral stereocenters. The chiral axis is viewed end-on and the two "near" and two "far" substituents on the axial unit are ranked, but with the additional rule that the two near substituents have higher priority than the far ones.

Tröger's base is a white solid tetracyclic organic compound. structure and formula of (CH3C6H3NCH2)2CH2. Tröger's base and its analogs are soluble in various organic solvents and strong acidic aqueous solutions due to their protonation.
Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.

In stereochemistry, asymmetric induction describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.
In stereochemistry, topicity is the stereochemical relationship between substituents and the structure to which they are attached. Depending on the relationship, such groups can be heterotopic, homotopic, enantiotopic, or diastereotopic.

Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonded to four different substituents. This type of construction creates two possible enantiomers. Absolute configuration uses a set of rules to describe the relative positions of each bond around the chiral center atom. The most common labeling method uses the descriptors R or S is based on the Cahn–Ingold–Prelog priority rules. R and S refer to Rectus and Sinister, which are Latin for right and left, respectively.

Chirality is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek χειρ (kheir), "hand", a familiar chiral object.
In chemistry, pyramidal inversion is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion passing through a planar transition state. For a compound that would otherwise be chiral due to a stereocenter, pyramidal inversion allows its enantiomers to racemize.

P-Chiral phosphines are organophosphorus compounds of the formula PRR′R″, where R, R′, R″ = H, alkyl, aryl, etc. They are a subset of chiral phosphines, a broader class of compounds where the stereogenic center can reside at sites other than phosphorus. P-chirality exploits the high barrier for inversion of phosphines, which ensures that enantiomers of PRR'R" do not racemize readily. The inversion barrier is relatively insensitive to substituents for triorganophosphines. By contrast, most amines of the type NRR′R″ undergo rapid pyramidal inversion.
Chemical compounds that come as mirror-image pairs are referred to by chemists as chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mixture of enantiomers are called racemic drugs and these are obviously devoid of optical rotation. The most commonly encountered stereogenic unit, that confers chirality to drug molecules are stereogenic center. Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S). The word chiral describes the three-dimensional architecture of the molecule and does not reveal the stereochemical composition. Hence "chiral drug" does not say whether the drug is racemic, single enantiomer or some other combination of stereoisomers. To resolve this issue Joseph Gal introduced a new term called unichiral. Unichiral indicates that the stereochemical composition of a chiral drug is homogenous consisting of a single enantiomer.