Related Research Articles
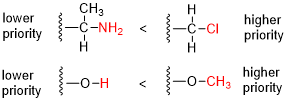
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of stereocenters will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands; without mirroring one of them, hands cannot be superposed onto each other. No amount of reorientation in three spatial dimensions will allow the four unique groups on the chiral carbon to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers.

In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups creates a new stereoisomer. Stereocenters are also referred to as stereogenic centers.
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.

In geometry, a figure is chiral if it is not identical to its mirror image, or, more precisely, if it cannot be mapped to its mirror image by rotations and translations alone. An object that is not chiral is said to be achiral.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.

The term supermolecule was introduced by Karl Lothar Wolf et al. (Übermoleküle) in 1937 to describe hydrogen-bonded acetic acid dimers. The study of non-covalent association of complexes of molecules has since developed into the field of supramolecular chemistry. The term supermolecule is sometimes used to describe supramolecular assemblies, which are complexes of two or more molecules that are not covalently bonded. The term supermolecule is also used in biochemistry to describe complexes of biomolecules, such as peptides and oligonucleotides composed of multiple strands.
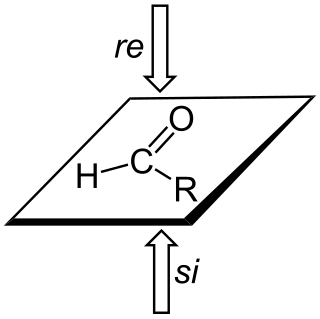
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.

Supramolecular electronics is the experimental field of supramolecular chemistry that bridges the gap between molecular electronics and bulk plastics in the construction of electronic circuitry at the nanoscale 1. In supramolecular electronics, assemblies of pi-conjugated systems on the 5 to 100 nanometer length scale are prepared by molecular self-assembly with the aim to fit these structures between electrodes. With single-molecules as researched in molecular electronics at the 5 nanometer scale this would be impractical. Nanofibers can be prepared from polymers such as polyaniline and polyacetylene 12. Chiral oligo(p-phenylenevinylene)s self-assemble in a controlled fashion into (helical) wires 3. An example of actively researched compounds in this field are certain coronenes.
The term "polymer" refers to large molecules whose structure is composed of multiple repeating units and the prefix "supra" meaning "beyond the limits of". Supramolecular polymers are a new category of polymers that can potentially be used for material applications beyond the limits of conventional polymers. By definition, supramolecular polymers are polymeric arrays of monomeric units that are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bonding, chalcogen bonding, and host–guest interaction. The direction and strength of the interactions are precisely tuned so that the array of molecules behaves as a polymer in dilute and concentrated solution, as well as in the bulk.
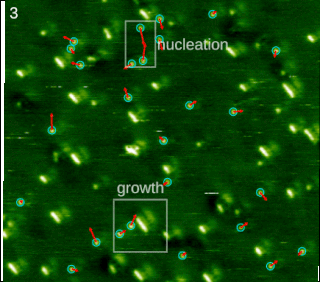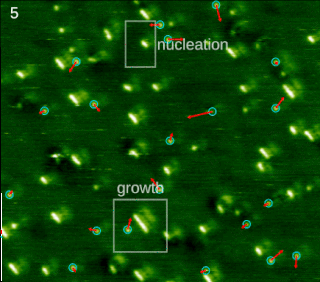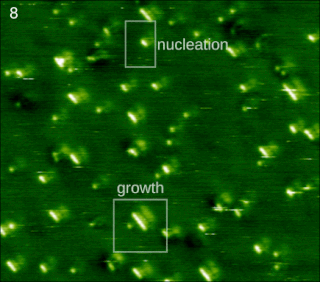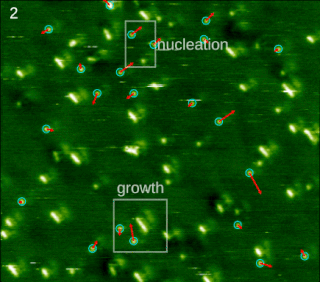
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intermolecular and intramolecular. Commonly, the term molecular self-assembly refers to the former, while the latter is more commonly called folding.

Takuzo Aida is a polymer chemist known for his work in the fields of supramolecular chemistry, materials chemistry and polymer chemistry. Aida, who is the Deputy Director for the RIKEN Center for Emergent Matter Science (CEMS) and a Distinguished University Professor at the University of Tokyo, has made pioneering contributions to the initiation, fundamental progress, and conceptual expansion of supramolecular polymerization. Aida has also been a leader and advocate for addressing critical environmental issues caused by plastic waste and microplastics in the oceans, soil, and food supply, through the development of dynamic, responsive, healable, reorganizable, and adaptive supramolecular polymers and related soft materials.

In chemistry, inherent chirality is a property of asymmetry in molecules arising, not from a stereogenic or chiral center, but from a twisting of the molecule in 3-D space. The term was first coined by Volker Boehmer in a 1994 review, to describe the chirality of calixarenes arising from their non-planar structure in 3-D space.

Egbert (Bert) Willem Meijer is a Dutch organic chemist, known for his work in the fields of supramolecular chemistry, materials chemistry and polymer chemistry. Meijer, who is distinguished professor of Molecular Sciences at Eindhoven University of Technology (TU/e) and Academy Professor of the Royal Netherlands Academy of Arts and Sciences, is considered one of the founders of the field of supramolecular polymer chemistry. Meijer is a prolific author, sought-after academic lecturer and recipient of multiple awards in the fields of organic and polymer chemistry.

Chirality is a property of asymmetry important in several branches of science. The word chirality is derived from the Greek χειρ (kheir), "hand", a familiar chiral object.

Supramolecular catalysis is not a well-defined field but it generally refers to an application of supramolecular chemistry, especially molecular recognition and guest binding, toward catalysis. This field was originally inspired by enzymatic system which, unlike classical organic chemistry reactions, utilizes non-covalent interactions such as hydrogen bonding, cation-pi interaction, and hydrophobic forces to dramatically accelerate rate of reaction and/or allow highly selective reactions to occur. Because enzymes are structurally complex and difficult to modify, supramolecular catalysts offer a simpler model for studying factors involved in catalytic efficiency of the enzyme. Another goal that motivates this field is the development of efficient and practical catalysts that may or may not have an enzyme equivalent in nature.

Subi Jacob George is an Indian organic chemist, known for his work in the fields of supramolecular chemistry, materials chemistry, and polymer chemistry. His research interests includes organic and supramolecular synthesis, functional organic materials, supramolecular polymers, chiral amplification, hybrid materials, and optoelectronic materials.

Roeland J. M. Nolte is a Dutch chemist, known for his work in the fields of organic chemistry, biochemistry, polymer chemistry, and supramolecular chemistry. He is an emeritus Royal Netherlands of Arts and Sciences professor and an emeritus professor of Organic Chemistry at Radboud University in Nijmegen, The Netherlands. Currently, he holds a special chair, i.e. professor of Molecular Nanotechnology, at this university. Nolte is considered to be one of the pioneers of the field of supramolecular chemistry, which encompasses the design and synthesis of new chemical structures from low molecular weight compounds and biopolymers using so-called non-covalent interactions. He published many studies on supramolecular assembly and biomimetic catalysts, which find applications in the field of nanomaterials and medicine.
When Topology Meets Chemistry: A Topological Look At Molecular Chirality is a book in chemical graph theory on the graph-theoretic analysis of chirality in molecular structures. It was written by Erica Flapan, based on a series of lectures she gave in 1996 at the Institut Henri Poincaré, and was published in 2000 by the Cambridge University Press and Mathematical Association of America as the first volume in their shared Outlooks book series.
Magalí Lingenfelder is an Argentinian chemist who is head of the Max Planck Laboratory for Molecular Nanoscience in École Polytechnique Fédérale de Lausanne. Her work looks to control atomic interfaces for energy conversion and antimicrobial surfaces. She was awarded the Max Planck Society Otto Hahn Medal in 2008.
References
- ↑ Suárez M, Branda N, Lehn JM, Decian A, Fischer J (1998). "Supramolecular Chirality: Chiral hydrogen-bonded supermolecules from achiral molecular components". Helvetica Chimica Acta. 81: 1–13. doi:10.1002/hlca.19980810102.