Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequencess.

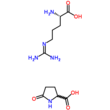
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid.

Post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes translating mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.

Arginase (EC 3.5.3.1, arginine amidinase, canavanase, L-arginase, arginine transamidinase) is a manganese-containing enzyme. The reaction catalyzed by this enzyme is:
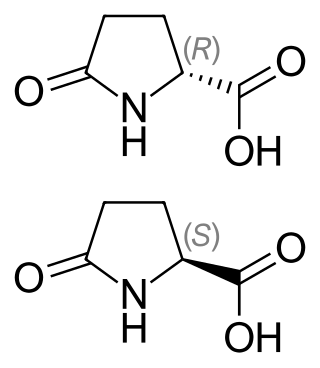
Pyroglutamic acid is a ubiquitous but understudied natural amino acid derivative in which the free amino group of glutamic acid or glutamine cyclizes to form a lactam. The names of pyroglutamic acid conjugate base, anion, salts, and esters are pyroglutamate, 5-oxoprolinate, or pidolate.

Argininosuccinate synthase or synthetase is an enzyme that catalyzes the synthesis of argininosuccinate from citrulline and aspartate. In humans, argininosuccinate synthase is encoded by the ASS gene located on chromosome 9.

The enzyme argininosuccinate lyase (EC 4.3.2.1, ASL, argininosuccinase; systematic name 2-(N ω-L-arginino)succinate arginine-lyase (fumarate-forming)) catalyzes the reversible breakdown of argininosuccinate:
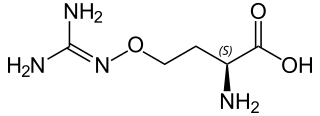
L-(+)-(S)-Canavanine is a non-proteinogenic amino acid found in certain leguminous plants. It is structurally related to the proteinogenic α-amino acid L-arginine, the sole difference being the replacement of a methylene bridge (-CH
2- unit) in arginine with an oxa group (i.e., an oxygen atom) in canavanine. Canavanine is accumulated primarily in the seeds of the organisms which produce it, where it serves both as a highly deleterious defensive compound against herbivores (due to cells mistaking it for arginine) and a vital source of nitrogen for the growing embryo. The related L-canaline is similar to ornithine.

Asymmetric dimethylarginine (ADMA) is a naturally occurring chemical found in blood plasma. It is a metabolic by-product of continual protein modification processes in the cytoplasm of all human cells. It is closely related to L-arginine, a conditionally essential amino acid. ADMA interferes with L-arginine in the production of nitric oxide (NO), a key chemical involved in normal endothelial function and, by extension, cardiovascular health.
N-Acetylglutamate synthase (NAGS) is an enzyme that catalyses the production of N-acetylglutamate (NAG) from glutamate and acetyl-CoA.

L-Arginine:glycine amidinotransferase is the enzyme that catalyses the transfer of an amidino group from L-arginine to glycine. The products are L-ornithine and glycocyamine, also known as guanidinoacetate, the immediate precursor of creatine. Creatine and its phosphorylated form play a central role in the energy metabolism of muscle and nerve tissues. Creatine is in highest concentrations in the skeletal muscle, heart, spermatozoa and photoreceptor cells. Creatine helps buffer the rapid changes in ADP/ATP ratio in muscle and nerve cells during active periods. Creatine is also synthesized in other tissues, such as pancreas, kidneys, and liver, where amidinotransferase is located in the cytoplasm, including the intermembrane space of the mitochondria, of the cells that make up those tissues.
CARM1, also known as PRMT4, is an enzyme encoded by the CARM1 gene found in human beings, as well as many other mammals. It has a polypeptide (L) chain type that is 348 residues long, and is made up of alpha helices and beta sheets. Its main function includes catalyzing the transfer of a methyl group from S-Adenosyl methionine to the side chain nitrogens of arginine residues within proteins to form methylated arginine derivatives and S-Adenosyl-L-homocysteine. CARM1 is a secondary coactivator through its association with p160 family of coactivators. It is responsible for moving cells toward the inner cell mass in developing blastocysts.

In enzymology, an alanine racemase is an enzyme that catalyzes the chemical reaction

The enzyme Acid-Induced Arginine Decarboxylase (AdiA), also commonly referred to as arginine decarboxylase, catalyzes the conversion of L-arginine into agmatine and carbon dioxide. The process consumes a proton in the decarboxylation and employs a pyridoxal-5'-phosphate (PLP) cofactor, similar to other enzymes involved in amino acid metabolism, such as ornithine decarboxylase and glutamine decarboxylase. It is found in bacteria and virus, though most research has so far focused on forms of the enzyme in bacteria. During the AdiA catalyzed decarboxylation of arginine, the necessary proton is consumed from the cell cytoplasm which helps to prevent the over-accumulation of protons inside the cell and serves to increase the intracellular pH. Arginine decarboxylase is part of an enzymatic system in Escherichia coli, Salmonella Typhimurium, and methane-producing bacteria Methanococcus jannaschii that makes these organisms acid resistant and allows them to survive under highly acidic medium.

In enzymology, an arginine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a tyrosine—arginine ligase is an enzyme that catalyzes the chemical reaction

In enzymology, a protein-arginine deiminase (EC 3.5.3.15) is an enzyme that catalyzes a form of post translational modification called arginine de-imination or citrullination:
In enzymology, a NAD(P)+-protein-arginine ADP-ribosyltransferase (EC 2.4.2.31) is an enzyme that catalyzes the chemical reaction using nicotinamide adenine dinucleotide

l-Arginine ethyl ester or ethyl arginate is an alternative supplement form of the conditionally-essential amino acid arginine bound to an ethyl ester. Esters are organic compounds formed by esterification – the reaction of carboxylic acid and alcohols.
Arginine and proline metabolism is one of the central pathways for the biosynthesis of the amino acids arginine and proline from glutamate. The pathways linking arginine, glutamate, and proline are bidirectional. Thus, the net utilization or production of these amino acids is highly dependent on cell type and developmental stage. Altered proline metabolism has been linked to metastasis formation in breast cancer.