Related Research Articles

A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.

A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.

The epidermal growth factor receptor is a transmembrane protein that is a receptor for members of the epidermal growth factor family of extracellular protein ligands.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.

Lapatinib (INN), used in the form of lapatinib ditosylate (USAN) is an orally active drug for breast cancer and other solid tumours. It is a dual tyrosine kinase inhibitor which interrupts the HER2/neu and epidermal growth factor receptor (EGFR) pathways. It is used in combination therapy for HER2-positive breast cancer. It is used for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 (ErbB2).

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.

Signal transducer and activator of transcription 5 (STAT5) refers to two highly related proteins, STAT5A and STAT5B, which are part of the seven-membered STAT family of proteins. Though STAT5A and STAT5B are encoded by separate genes, the proteins are 90% identical at the amino acid level. STAT5 proteins are involved in cytosolic signalling and in mediating the expression of specific genes. Aberrant STAT5 activity has been shown to be closely connected to a wide range of human cancers, and silencing this aberrant activity is an area of active research in medicinal chemistry.

Cluster of differentiation antigen 135 (CD135) also known as fms like tyrosine kinase 3, receptor-type tyrosine-protein kinase FLT3, or fetal liver kinase-2 (Flk2) is a protein that in humans is encoded by the FLT3 gene. FLT3 is a cytokine receptor which belongs to the receptor tyrosine kinase class III. CD135 is the receptor for the cytokine Flt3 ligand (FLT3L).

Fibroblast growth factor receptor 1 (FGFR1), also known as basic fibroblast growth factor receptor 1, fms-related tyrosine kinase-2 / Pfeiffer syndrome, and CD331, is a receptor tyrosine kinase whose ligands are specific members of the fibroblast growth factor family. FGFR1 has been shown to be associated with Pfeiffer syndrome, and clonal eosinophilias.
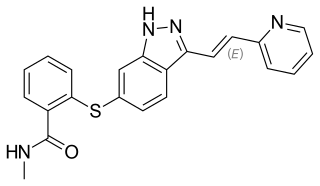
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.

Proto-oncogene tyrosine-protein kinase ROS is an enzyme that in humans is encoded by the ROS1 gene.

Omacetaxine mepesuccinate, formerly named as homoharringtonine or HHT, is a pharmaceutical drug substance that is indicated for treatment of chronic myeloid leukemia (CML).
A CDK inhibitor is any chemical that inhibits the function of CDKs. They are used to treat cancers by preventing overproliferation of cancer cells. The US FDA approved the first drug of this type, palbociclib (Ibrance), a CDK4/6 inhibitor, in February 2015, for use in postmenopausal women with breast cancer that is estrogen receptor positive and HER2 negative. While there are multiple cyclin/CDK complexes regulating the cell cycle, CDK inhibitors targeting CDK4/6 have been the most successful, with 4 CDK4/6 inhibitors haven been FDA approved. No inhibitors targeting other CDKs have been FDA approved, but several compounds are in clinical trials.

A tyrosine kinase inhibitor (TKI) is a pharmaceutical drug that inhibits tyrosine kinases. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. The proteins are activated by adding a phosphate group to the protein (phosphorylation), a step that TKIs inhibit. TKIs are typically used as anticancer drugs. For example, they have substantially improved outcomes in chronic myelogenous leukemia. They have also been used to treat other diseases, such as idiopathic pulmonary fibrosis.

Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC). It acts as an ALK and ROS1 inhibitor.
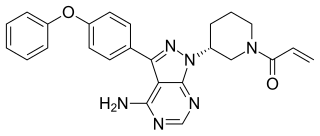
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that inhibits B-cell proliferation and survival by irreversibly binding the protein Bruton's tyrosine kinase (BTK). Blocking BTK inhibits the B-cell receptor pathway, which is often aberrantly active in B cell cancers. Ibrutinib is therefore used to treat such cancers, including mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia. Ibrutinib also binds to C-terminal Src Kinases. These are off-target receptors for the BTK inhibitor. Ibrutinib binds to these receptors and inhibits the kinase from promoting cell differentiation and growth. This leads to many different side effects like left atrial enlargement and atrial fibrillation during the treatment of Chronic Lymphocytic Leukemia.

mTOR inhibitors are a class of drugs that inhibit the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.

Osimertinib, sold under the brand name Tagrisso, is a medication used to treat non-small-cell lung carcinomas with specific mutations. It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.
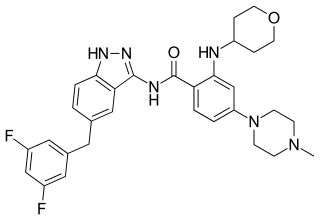
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).
Craig M. Crews is an American scientist at Yale University known for his contributions to chemical biology. He is known for his contributions to the field of induced proximity through his work in creating heterobifunctional molecules that hijack cellular processes by inducing the interaction of two proteins inside a living cell. His initial work focused on the discovery of PROteolysis-TArgeting Chimeras (PROTACs) to trigger degradation of disease-causing proteins, a process known as targeted protein degradation (TPD), and he has since developed new versions of -TACs to leverage other cellular processes and protein families to treat disease.
References
- ↑ Gross S, Rahal R, Stransky N, Lengauer C, Hoeflich KP (May 2015). "Targeting cancer with kinase inhibitors". The Journal of Clinical Investigation. 125 (5): 1780–1789. doi:10.1172/JCI76094. PMC 4463189 . PMID 25932675.
- ↑ "Definition of tyrosine kinase inhibitor - NCI Dictionary of Cancer Terms". Archived from the original on 2008-05-11.
- ↑ Jänne, Pasi A.; Gray, Nathanael; Settleman, Jeff (2009). "Factors underlying sensitivity of cancers to small-molecule kinase inhibitors". Nature Reviews Drug Discovery. 8 (9): 709–23. doi:10.1038/nrd2871. PMID 19629074. S2CID 7817325.
- ↑ "Clinical trials using WEE1 inhibitor AZD1775". National Cancer Institute. Retrieved April 20, 2018.
- ↑ "Janssen announces U.S. FDA breakthrough therapy designation for erdafitinib in the Treatment of metastatic urothelial cancer". Johnson and Johnson. March 15, 2018. Retrieved April 20, 2018.
- ↑ Bajpai, M (2009). "Fostamatinib, a Syk inhibitor prodrug for the treatment of inflammatory diseases". IDrugs. 12 (3): 174–85. PMID 19333898.
- ↑ "FDA Grants Imatinib (Gleevec) Full Approval for Adjuvant Treatment of GIST".
- ↑ "Medscape Multispecialty – Home page". WebMD . Retrieved 27 November 2013.[ full citation needed ]
- ↑ Monograph
- ↑ "European Public Assessment Reports". European Medicines Agency . Retrieved 27 January 2014.[ full citation needed ]
- ↑ "Therapeutic Goods Administration – Home page". Department of Health (Australia) . Retrieved 27 November 2013.[ full citation needed ]