Related Research Articles

Piezoelectricity is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from Greek πιέζειν (piezein) 'to squeeze/press', and ἤλεκτρον (ēlektron) 'amber'.
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Joseph Valasek. Thus, the prefix ferro, meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric and ferromagnetic are known as multiferroics.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.
In chemistry, titanate usually refers to inorganic compounds composed of titanium oxides. Together with niobate, titanate salts form the Perovskite group.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
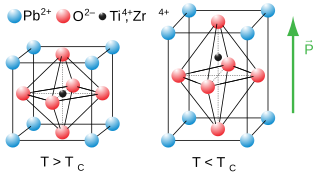
Lead zirconate titanate, also called lead zirconium titanate and commonly abbreviated as PZT, is an inorganic compound with the chemical formula Pb[ZrxTi1−x]O3(0 ≤ x ≤ 1). It is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. It is a white to off-white solid.
Multiferroics are defined as materials that exhibit more than one of the primary ferroic properties in the same phase:

Lanthanum(III) oxide, also known as lanthana, chemical formula La2O3, is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses.

Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics.

Bismuth germanium oxide or bismuth germanate is an inorganic chemical compound of bismuth, germanium and oxygen. Most commonly the term refers to the compound with chemical formula Bi4Ge3O12 (BGO), with the cubic evlitine crystal structure, used as a scintillator. (The term may also refer to a different compound with formula Bi12GeO20, an electro-optical material with sillenite structure, and Bi2Ge3O9.)
Bismuth ferrite (BiFeO3, also commonly referred to as BFO in materials science) is an inorganic chemical compound with perovskite structure and one of the most promising multiferroic materials. The room-temperature phase of BiFeO3 is classed as rhombohedral belonging to the space group R3c. It is synthesized in bulk and thin film form and both its antiferromagnetic (G type ordering) Néel temperature (approximately 653 K) and ferroelectric Curie temperature are well above room temperature (approximately 1100K). Ferroelectric polarization occurs along the pseudocubic direction () with a magnitude of 90–95 μC/cm2.

Lead(II) titanate is an inorganic compound with the chemical formula PbTiO3. It is the lead salt of titanic acid. Lead(II) titanate is a yellow powder that is insoluble in water.
Aurivillius phases are a form of perovskite represented by the general formulae is (Bi2O2)(An−1BnO3n+1) (where A is a large 12 co-ordinate cation, and B is a small 6 co-ordinate cation).
Relaxor ferroelectrics are ferroelectric materials that exhibit high electrostriction. As of 2015, although they have been studied for over fifty years, the mechanism for this effect is still not completely understood, and is the subject of continuing research.

Bismuth titanate or bismuth titanium oxide is a solid inorganic compound of bismuth, titanium and oxygen with the chemical formula of Bi12TiO20, Bi 4Ti3O12 or Bi2Ti2O7.
A polar metal, metallic ferroelectric, or ferroelectric metal is a metal that contains an electric dipole moment. Its components have an ordered electric dipole. Such metals should be unexpected, because the charge should conduct by way of the free electrons in the metal and neutralize the polarized charge. However they do exist. Probably the first report of a polar metal was in single crystals of the cuprate superconductors YBa2Cu3O7−δ,. A polarization was observed along one (001) axis by pyroelectric effect measurements, and the sign of the polarization was shown to be reversible, while its magnitude could be increased by poling with an electric field. The polarization was found to disappear in the superconducting state. The lattice distortions responsible were considered to be a result of oxygen ion displacements induced by doped charges that break inversion symmetry. The effect was utilized for fabrication of pyroelectric detectors for space applications, having the advantage of large pyroelectric coefficient and low intrinsic resistance. Another substance family that can produce a polar metal is the nickelate perovskites. One example interpreted to show polar metallic behavior is lanthanum nickelate, LaNiO3. A thin film of LaNiO3 grown on the (111) crystal face of lanthanum aluminate, (LaAlO3) was interpreted to be both conductor and a polar material at room temperature. The resistivity of this system, however, shows an upturn with decreasing temperature, hence does not strictly adhere to the definition of a metal. Also, when grown 3 or 4 unit cells thick (1-2 nm) on the (100) crystal face of LaAlO3, the LaNiO3 can be a polar insulator or polar metal depending on the atomic termination of the surface. Lithium osmate, LiOsO3 also undergoes a ferrorelectric transition when it is cooled below 140K. The point group changes from R3c to R3c losing its centrosymmetry. At room temperature and below, lithium osmate is an electric conductor, in single crystal, polycrystalline or powder forms, and the ferroelectric form only appears below 140K. Above 140K the material behaves like a normal metal. Artificial two-dimensional polar metal by charge transfer to a ferroelectric insulator has been realized in LaAlO3/Ba0.8Sr0.2TiO3/SrTiO3 complex oxide heterostructures.

Bengt Aurivillius was a Swedish chemist known for his research in metal and mixed oxides.

Dragan Damjanovic is a Swiss-Bosnian-Herzegovinian materials scientist. From 2008 to 2022, he was a professor of material sciences at EPFL and head of the Group for Ferroelectrics and Functional Oxides.
Nickel niobate is a complex oxide which as a solid material has found potential applications in catalysis and lithium batteries.
References
- ↑ Smolenskii, G.; Isupov, V.; Agranovskaya, A.; Krainik, N. (1961). "New ferroelectrics of complex composition". Sov. Phys. Solid State. 2: 2651–2654.
- ↑ Zvirgzds, J.A.; Kapostin, P.P.; Zvirgzde, J.V.; Kruzina, T.V. (1982). "X-ray study of phase transitions in ferroelectric Na0.5Bi0.5TiO3". Ferroelectrics. 40 (1): 75–77. Bibcode:1982Fer....40...75Z. doi:10.1080/00150198208210600.
- ↑ Bousquet, M.; Duclere, J.R.; Orhan, E.; Boulle, A.; Bachelet, C.; Champeaux, C (2010). "Optical properties of an epitaxial Na0.5Bi0.5TiO3 thin film grown by laser ablation: Experimental approach and density functional theory calculations". J. Appl. Phys. 107 (10): 104107–104107–13. Bibcode:2010JAP...107j4107B. doi:10.1063/1.3400095.
- ↑ Takenaka, T.; Maruyama, K.-I.; Sakata, K. (1991). "(Bi1/2Na1/2)TiO3-BaTiO3 system for lead-free piezoelectric ceramics". Jpn. J. Appl. Phys. Part 1. 30 (9S): 2236–2239. Bibcode:1991JaJAP..30.2236T. doi:10.1143/JJAP.30.2236. S2CID 124093028.
- ↑ Sasaki, A.; Chiba, T.; Mamiya, Y.; Otsuki, E. Dielectric and piezoelectric properties of (Bi0.5Na0.5)TiO3-(Bi0.5K0.5)TiO3 systems. Jpn. J. Appl. Phys. Part 1 1999, 38, 5564–5567.
- ↑ Reichmann, K.; Feteira, A., Li M. (2015). "Bismuth Sodium Titanate Based Materials for Piezoelectric Actuators". Materials. 8 (12): 8467–8495. Bibcode:2015Mate....8.8467R. doi: 10.3390/ma8125469 . PMC 5458809 . PMID 28793724.
{{cite journal}}: CS1 maint: multiple names: authors list (link)