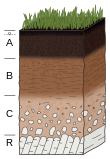
Soil formation, also known as pedogenesis, is the process of soil genesis as regulated by the effects of place, environment, and history. Biogeochemical processes act to both create and destroy order (anisotropy) within soils. These alterations lead to the development of layers, termed soil horizons, distinguished by differences in color, structure, texture, and chemistry. These features occur in patterns of soil type distribution, forming in response to differences in soil forming factors.

Oxisols are a soil order in USDA soil taxonomy, best known for their occurrence in tropical rain forest within 25 degrees north and south of the Equator. In the World Reference Base for Soil Resources (WRB), they belong mainly to the ferralsols, but some are plinthosols or nitisols. Some oxisols have been previously classified as laterite soils.
A soil horizon is a layer parallel to the soil surface whose physical, chemical and biological characteristics differ from the layers above and beneath. Horizons are defined in many cases by obvious physical features, mainly colour and texture. These may be described both in absolute terms and in terms relative to the surrounding material, i.e. 'coarser' or 'sandier' than the horizons above and below.

Bioturbation is defined as the reworking of soils and sediments by animals or plants. It includes burrowing, ingestion, and defecation of sediment grains. Bioturbating activities have a profound effect on the environment and are thought to be a primary driver of biodiversity. The formal study of bioturbation began in the 1800s by Charles Darwin experimenting in his garden. The disruption of aquatic sediments and terrestrial soils through bioturbating activities provides significant ecosystem services. These include the alteration of nutrients in aquatic sediment and overlying water, shelter to other species in the form of burrows in terrestrial and water ecosystems, and soil production on land.

Bioirrigation refers to the process of benthic organisms flushing their burrows with overlying water. The exchange of dissolved substances between the porewater and overlying seawater that results is an important process in the context of the biogeochemistry of the oceans.

In oceanography and limnology, the sediment–water interface is the boundary between bed sediment and the overlying water column. The term usually refers to a thin layer of water at the very surface of sediments on the seafloor. In the ocean, estuaries, and lakes, this layer interacts with the water above it through physical flow and chemical reactions mediated by the micro-organisms, animals, and plants living at the bottom of the water body. The topography of this interface is often dynamic, as it is affected by physical processes and biological processes. Physical, biological, and chemical processes occur at the sediment-water interface as a result of a number of gradients such as chemical potential gradients, pore water gradients, and oxygen gradients.
Claypan is a dense, compact, slowly permeable layer in the subsoil. It has a much higher clay content than the overlying material, from which it is separated by a sharply defined boundary. The dense structure restricts root growth and water infiltration. Therefore, a perched water table might form on top of the claypan. In the Canadian classification system, claypan is defined as a clay-enriched illuvial B (Bt) horizon.

A fossorial animal is one that is adapted to digging and which lives primarily underground. Examples of fossorial vertebrates are badgers, naked mole-rats, meerkats, and mole salamanders. Among invertebrates, many molluscs, insects, and arachnids are fossorial.

Mima mounds are low, flattened, circular to oval, domelike, natural mounds that are composed of loose, unstratified, often gravelly sediment that is an overthickened A horizon. These mounds range in diameter from 3 m (9.8 ft) to more than 50 m (160 ft); in height 30 cm (12 in) to greater than 2 m (6.6 ft); and in density from several to greater than 50 mounds per hectare, at times forming conspicuous natural patterns. Mima mounds can be seen at the Mima Mounds Natural Area Preserve in Washington state.

Soil morphology is the branch of soil science dedicated to the technical description of soil, particularly physical properties including texture, color, structure, and consistence. Morphological evaluations of soil are typically performed in the field on a soil profile containing multiple horizons.

Soil biology is the study of microbial and faunal activity and ecology in soil. Soil life, soil biota, soil fauna, or edaphon is a collective term that encompasses all organisms that spend a significant portion of their life cycle within a soil profile, or at the soil-litter interface. These organisms include earthworms, nematodes, protozoa, fungi, bacteria, different arthropods, as well as some reptiles, and species of burrowing mammals like gophers, moles and prairie dogs. Soil biology plays a vital role in determining many soil characteristics. The decomposition of organic matter by soil organisms has an immense influence on soil fertility, plant growth, soil structure, and carbon storage. As a relatively new science, much remains unknown about soil biology and its effect on soil ecosystems.
The early concepts of soil were based on ideas developed by a German chemist, Justus von Liebig (1803–1873), and modified and refined by agricultural scientists who worked on samples of soil in laboratories, greenhouses, and on small field plots. The soils were rarely examined below the depth of normal tillage. These chemists held the "balance-sheet" theory of plant nutrition. Soil was considered a more or less static storage bin for plant nutrients—the soils could be used and replaced. This concept still has value when applied within the framework of modern soil science, although a useful understanding of soils goes beyond the removal of nutrients from soil by harvested crops and their return in manure, lime, and fertilizer.

Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles either have their origins in soil and rocks and have been transported from the land to the sea, mainly by rivers but also by dust carried by wind and by the flow of glaciers into the sea, or they are biogenic deposits from marine organisms or from chemical precipitation in seawater, as well as from underwater volcanoes and meteorite debris.

Heuweltjies are large mounds above or near the surface of the landscape, a type of soil surface feature that occurs widely in the south-western Cape of South Africa. Their formation has been the subject of a wide range of speculation and of debate.

An earthworm is a soil-dwelling terrestrial invertebrate that belongs to the phylum Annelida. The term is the common name for the largest members of the class Oligochaeta. In classical systems, they were in the order of Opisthopora since the male pores opened posterior to the female pores, although the internal male segments are anterior to the female. Theoretical cladistic studies have placed them in the suborder Lumbricina of the order Haplotaxida, but this may change. Other slang names for earthworms include "dew-worm", "rainworm", "nightcrawler", and "angleworm". Larger terrestrial earthworms are also called megadriles as opposed to the microdriles in the semiaquatic families Tubificidae, Lumbricidae and Enchytraeidae. The megadriles are characterized by a distinct clitellum and a vascular system with true capillaries.

Plant litter is dead plant material that have fallen to the ground. This detritus or dead organic material and its constituent nutrients are added to the top layer of soil, commonly known as the litter layer or O horizon. Litter is an important factor in ecosystem dynamics, as it is indicative of ecological productivity and may be useful in predicting regional nutrient cycling and soil fertility.
A stonelayer, or soil stonelayer, or stone line, is a three-dimensional subsurface layer, or soil horizon, dominated by coarse particles (>2mm), that generally follows (mimics) the surface topography. A stonelayer occupies the basal horizon of two-layered soil biomantles. A stonelayer may be one stone thick, and thus appear in a trench or pit as a "stone line," or it may be several stones thick and appear as a "stone zone". The gravel components of stonelayers may be compositionally variable, and while many are lithic clasts, often of quartzose composition, others may be metallic nodules and concretions of iron and manganese oxides, human artifacts, snail and clam shells, precious and semi-precious stones, or some combination thereof.
A stone line is a three-dimensional subsurface layer, or ‘carpet,’ of stones evident as a ‘line of stones’ in natural exposures such as soils, road cuts, and trenches. Stone lines that are more than one stone thick have been called ‘stone zones’. Stone lines and stone zones are known to occur in soils, paleosols, and in non-soil geologic-stratigraphic sequences. Where present in stratigraphic sequences, if the units were deposited by running water, the stones are usually imbricated. This is strong evidence that such stone lines are geogenic. On the other hand, a stone line that is present in a soil or a paleosol is invariable non-imbricated and follows (mimics) the surface topography of the soil, or the paleosurface of a paleosol. This is strong evidence that such stone lines are pedogenic, and produced by soil forming processes. As it turns out, experience has shown that most stone lines are indeed associated with soils and paleosols, and most are consequently assumed to be pedogenic. How stone lines form, whether geogenically by geologic processes or pedogenically by soil forming processes is invariably a matter of interpretation. And whether the interpretation is geogenic or pedogenic often reflects the background and training of the interpreter.
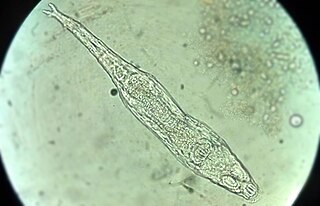
Soil mesofauna are invertebrates between 0.1mm and 2mm in size, which live in the soil or in a leaf litter layer on the soil surface. Members of this group include nematodes, mites, springtails (collembola), proturans, pauropods, rotifers, earthworms, tardigrades, small spiders, pseudoscorpions, opiliones (harvestmen), enchytraeidae such as potworms, insect larvae, small isopods and myriapods. They play an important part in the carbon cycle and are likely to be adversely affected by climate change.
Cultural layer is a key concept in archaeology, particularly culture-historical archaeology especially in archaeological digs or excavations. A cultural layer helps determine an archaeological culture: the remnants of human settlement that can be grouped and identified as coming from approximately the same distinct time period.