Dinocephalians are a clade of large-bodied early therapsids that flourished in the Early and Middle Permian between 279.5 and 260 million years ago (Ma), but became extinct during the Capitanian mass extinction event. Dinocephalians included herbivorous, carnivorous, and omnivorous forms. Many species had thickened skulls with many knobs and bony projections. Dinocephalians were the first non-mammalian therapsids to be scientifically described and their fossils are known from Russia, China, Brazil, South Africa, Zimbabwe, and Tanzania.

Pareiasaurs are an extinct clade of large, herbivorous parareptiles. Members of the group were armoured with osteoderms which covered large areas of the body. They first appeared in southern Pangea during the Middle Permian, before becoming globally distributed during the Late Permian. Pareiasaurs were the largest reptiles of the Permian, reaching sizes equivalent to those of contemporary therapsids. Pareiasaurs became extinct at the end of the Permian during the Permian-Triassic extinction event.

Diadectomorpha is a clade of large tetrapods that lived in Euramerica during the Carboniferous and Early Permian periods and in Asia during Late Permian (Wuchiapingian), They have typically been classified as advanced reptiliomorphs positioned close to, but outside of the clade Amniota, though some recent research has recovered them as the sister group to the traditional Synapsida within Amniota, based on inner ear anatomy and cladistic analyses. They include both large carnivorous and even larger herbivorous forms, some semi-aquatic and others fully terrestrial. The diadectomorphs seem to have originated during late Mississippian times, although they only became common after the Carboniferous rainforest collapse and flourished during the Late Pennsylvanian and Early Permian periods.

Sphenacodontidae is an extinct family of sphenacodontoid synapsids. Small to large, advanced, carnivorous, Late Pennsylvanian to middle Permian "pelycosaurs". The most recent one, Dimetrodon angelensis, is from the late Kungurian or early Roadian San Angelo Formation. However, given the notorious incompleteness of the fossil record, a recent study concluded that the Sphenacodontidae may have become extinct as recently as the early Capitanian. Primitive forms were generally small, but during the later part of the early Permian these animals grew progressively larger, to become the top predators of terrestrial environments. Sphenacodontid fossils are so far known only from North America and Europe.

Caseasauria is one of the two main clades of early synapsids, the other being the Eupelycosauria. Caseasaurs are currently known only from the Late Carboniferous and the Permian, and include two superficially different families, the small insectivorous or carnivorous Eothyrididae, and the large, herbivorous Caseidae. These two groups share a number of specialised features associated with the morphology of the snout and external naris.

Sphenacodontoidea is a node-based clade that is defined to include the most recent common ancestor of Sphenacodontidae and Therapsida and its descendants. Sphenacodontoids are characterised by a number of synapomorphies concerning proportions of the bones of the skull and the teeth.
Casea is a genus of herbivorous caseid synapsids that lived during the late Lower Permian (Kungurian) in what is now Texas, United States. The genus is only represented by its type species, Casea broilii, named by Samuel Wendell Williston in 1910. The species is represented by a skull associated with a skeleton, a second skull, a partial skull with a better preserved dentition than that of the preceding skulls, and several incomplete postcranial skeletons. Three other Casea species were later erected, but these are considered today to be invalid or belonging to different genera. Casea was a small animal with a length of about 1.20 m and a weight of around 20 kg.

Oedaleops is an extinct genus of caseasaur synapsids from the Early Permian of the southwestern United States. Fossils have been found in the Cutler Formation in New Mexico, which dates back to the Wolfcampian stage of the Early Permian. All remains belong to the single known species Oedaleops campi. Oedaleops was closely related to Eothyris, and both are part of the family Eothyrididae. Like Eothyris, it was probably an insectivore.

Cotylorhynchus is an extinct genus of herbivorous caseid synapsids that lived during the late Lower Permian (Kungurian) and possibly the early Middle Permian (Roadian) in what is now Texas and Oklahoma in the United States. The large number of specimens found make it the best-known caseid. Like all large herbivorous caseids, Cotylorhynchus had a short snout sloping forward and very large external nares. The head was very small compared to the size of the body. The latter was massive, barrel-shaped, and ended with a long tail. The limbs were short and robust. The hands and feet had short, broad fingers with powerful claws. The barrel-shaped body must have housed large intestines, suggesting that the animal had to feed on a large quantity of plants of low nutritional value. Caseids are generally considered to be terrestrial, though a semi-aquatic lifestyle has been proposed by some authors. The genus Cotylorhynchus is represented by three species, the largest of which could reach more than 6 m in length. However, a study published in 2022 suggests that the genus may be paraphyletic, with two of the three species possibly belonging to separate genera.
Angelosaurus is an extinct genus of herbivorous caseid synapsids that lived during the late Lower Permian (Kungurian) and early Middle Permian (Roadian) in what is now Texas and Oklahoma in the United States. Like other herbivorous caseids, it had a small head, large barrel-shaped body, long tail, and massive limbs. Angelosaurus differs from other caseids by the extreme massiveness of its bones, particularly those of the limbs, which show a strong development of ridges, processes, and rugosities for the attachment of muscles and tendons. Relative to its body size, the limbs of Angelosaurus were shorter and wider than those of other caseids. The ungual phalanges looked more like hooves than claws. The few known cranial elements show that the skull was short and more robust than that of the other representatives of the group. Angelosaurus is also distinguished by its bulbous teeth with shorter and wider crowns than those of other caseids. Their morphology and the high rate of wear they exhibit suggests a diet quite different from that of other large herbivorous caseids, and must have been based on particularly tough plants. A study published in 2022 suggests that the genus may be paraphyletic, with Angelosaurus possibly only represented by its type species A. dolani.

Ennatosaurus is an extinct genus of caseid synapsid that lived during the Middle Permian in northern European Russia. The genus is only represented by its type species, Ennatosaurus tecton, which was named in 1956 by Ivan Antonovich Efremov. The species is known from at least six skulls associated with their lower jaws, as well as from the postcranial bones of several juvenile individuals. Ennatosaurus has the typical caseid skull with a short snout tilted forward and very large external nares. However, it differs from other derived caseids by its postcranial skeleton with smaller proportions compared to the size of the skull. As with other advanced caseids, the teeth of Ennatosaurus were well suited for slicing and cutting vegetation. The presence of a highly developed hyoid apparatus indicates the presence of a massive and mobile tongue, which had to work in collaboration with the palatal teeth during swallowing. With a late Roadian - early Wordian age, Ennatosaurus is one of the last known caseids.

Mesenosaurus is an extinct genus of amniote. It belongs to the family Varanopidae. This genus includes two species: the type species Mesenosaurus romeri from the middle Permian Mezen River Basin of northern Russia, and Mesenosaurus efremovi from the early Permian (Artinskian) Richards Spur locality. M. romeri’s stratigraphic range is the middle to late Guadalupian while M. efremovi’s stratigraphic range is the Cisuralian.
Oromycter is an extinct genus of caseid synapsids from the Early Permian of Oklahoma. The sole and type species, Oromycter dolesorum, was named in 2005 by Robert R. Reisz.

Phreatophasma is an extinct genus of synapsids from the Middle Permian of European Russia. It includes only one species, Phreatophasma aenigmatum, which is itself known from a single femur found in a mine near the town of Belebei in Bashkortostan. Phreatophasma comes from a fossil assemblage that is latest Ufimian to earliest Kazanian in age under the Russian stratigraphic scheme, correlating with the Roadian Age under the international stratigraphic timescale. Because the species is based on a single specimen with few diagnostic anatomical features, uncertainty remains as to where it belongs in tetrapod phylogeny; originally interpreted in 1954 as an enigmatic "theromorph" synapsid by Soviet paleontologist Ivan Yefremov, Phreatophasma was later described as a therapsid incertae sedis by American paleontologist Alfred Romer in 1956 and then as a member of a basal synapsid family called Caseidae starting with Everett C. Olson in 1962. Olson's classification was later supported by Canadian paleontologist Robert Reisz in 1986 and American paleontologist Robert L. Carroll in 1988. Ivakhneneko et al. (1997) and Maddin et al. (2008) both considered Phreatophasma an indeterminate synapsid.

Callibrachion is an extinct genus of caseid synapsids that lived in east-central France during the Lower Permian (Asselian). The holotype and only known specimen (MNHN.F.AUT490) is represented by an almost complete postcranial skeleton associated with skull fragments discovered at the end of the 19th century in the Permian Autun basin in Saône-et-Loire department, in the Bourgogne-Franche-Comté region. It belongs to an immature individual measuring less than 1.50 m in length. Callibrachion was long considered a junior synonym of the genus Haptodus and classified among the sphenacodontid pelycosaurs. In 2015, a new study found that Callibrachion was a different animal from Haptodus and that it was a caseasaur rather than a sphenacodontid. This was confirmed in 2016 by a cladistic analysis which recovered Callibrachion as a basal caseid. Callibrachion's sharp teeth and unenlarged ribcage indicate that this animal was likely faunivorous.
Ruthenosaurus is an extinct genus of caseid synapsids that lived in what is now southern France during the Early Permian about 285 million years ago. It is known from the holotype MNHN.F.MCL-1 an articulated partial postcranial skeleton. It was collected by D. Sigogneau-Russell and D. Russell in 1970 in the upper part of the M2 Member, Grès Rouge Group, in the Rodez Basin, near the village of Valady, in Occitanie Region. It was first named by Robert R. Reisz, Hillary C. Maddin, Jörg Fröbisch and Jocelyn Falconnet in 2011, and the type species is Ruthenosaurus russellorum.

Euromycter is an extinct genus of caseid synapsids that lived in what is now southern France during the Early Permian about 285 million years ago. The holotype and only known specimen of Euromycter (MNHN.F.MCL-2) includes the complete skull with lower jaws and hyoid apparatus, six cervical vertebrae with proatlas, anterior part of interclavicle, partial right clavicle, right posterior coracoid, distal head of right humerus, left and right radius, left and right ulna, and complete left manus. It was collected by D. Sigogneau-Russell and D. Russell in 1970 at the top of the M1 Member, Grès Rouge Group, near the village of Valady, Rodez Basin. It was first assigned to the species "Casea" rutena by Sigogneau-Russell and Russell in 1974. More recently, it was reassigned to its own genus, Euromycter, by Robert R. Reisz, Hillary C. Maddin, Jörg Fröbisch and Jocelyn Falconnet in 2011. The preserved part of the skeleton suggests a size between 1,70 m (5,5 ft) and 1,80 m (5,9 ft) in length for this individual.
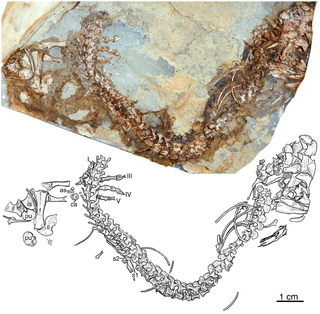
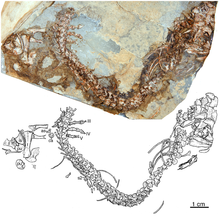
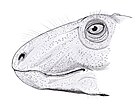
Eocasea is an extinct genus of caseid synapsids from the Late Pennsylvanian of Kansas. It is known from a single type species, Eocasea martini.

Alierasaurus is an extinct genus of caseid synapsid that lived during the early Middle Permian (Roadian) in what is now Sardinia. It is represented by a single species, the type species Alierasaurus ronchii. Known from a very large partial skeleton found within the Cala del Vino Formation, Alierasaurus is one of the largest known caseids. It closely resembles Cotylorhynchus, another giant caseid from the San Angelo Formation in Texas. The dimensions of the preserved foot elements and caudal vertebrae suggest an estimated total length of about 6 or 7 m for Alierasaurus. In fact, the only anatomical features that differ between Alierasaurus and Cotylorhynchus are found in the bones of the feet; Alierasaurus has a longer and thinner fourth metatarsal and it has ungual bones at the tips of the toes that are pointed and claw-like rather than flattened as in other caseids. Alierasaurus and Cotylorhynchus both have very wide, barrel-shaped rib cages indicating that they were herbivores that fed primarily on high-fiber plant material.

Lalieudorhynchus is an extinct genus of caseid synapsids that lived during the Guadalupian in what is now the south of France. The genus is only known by its type species, Lalieudorhynchus gandi, which was named in 2022 by Ralf Werneburg, Frederik Spindler, Jocelyn Falconnet, Jean-Sébastien Steyer, Monique Vianey-Liaud, and Joerg W. Schneider. Lalieudorhynchus is represented by a partial postcranial skeleton discovered in the Lodève basin in the central part of the Hérault department in the Occitanie region. It belongs to an individual measuring approximately 3.75 m (12.3 ft) in length. The degree of ossification of its bones, however, indicates that it was a late juvenile or still growing young adult. Based on the internal structure of its bones, the describing authors interpreted Lalieudorhynchus as a semiaquatic animal that may have had a lifestyle similar to that of hippopotamus, spending part of its time in water but returning to land for food, though the idea that caseids were semi-aquatic has been previously contested by other authors. It is geologically one of the youngest known representatives of the caseids. The phylogenetic analysis proposed by Werneburg and colleagues identified Lalieudorhynchus as a derived caseid closely related to the North American species "Cotylorhynchus" hancocki.