Magnetoencephalography (MEG) is a functional neuroimaging technique for mapping brain activity by recording magnetic fields produced by electrical currents occurring naturally in the brain, using very sensitive magnetometers. Arrays of SQUIDs are currently the most common magnetometer, while the SERF magnetometer is being investigated for future machines. Applications of MEG include basic research into perceptual and cognitive brain processes, localizing regions affected by pathology before surgical removal, determining the function of various parts of the brain, and neurofeedback. This can be applied in a clinical setting to find locations of abnormalities as well as in an experimental setting to simply measure brain activity.

Deep brain stimulation (DBS) is a surgical procedure that implants a neurostimulator and electrodes which sends electrical impulses to specified targets in the brain responsible for movement control. The treatment is designed for a range of movement disorders such as Parkinson's disease, essential tremor, and dystonia, as well as for certain neuropsychiatric conditions like obsessive-compulsive disorder (OCD) and epilepsy. The exact mechanisms of DBS are complex and not entirely clear, but it is known to modify brain activity in a structured way.

A brain–computer interface (BCI), sometimes called a brain–machine interface (BMI), is a direct communication pathway between the brain's electrical activity and an external device, most commonly a computer or robotic limb. BCIs are often directed at researching, mapping, assisting, augmenting, or repairing human cognitive or sensory-motor functions. They are often conceptualized as a human–machine interface that skips the intermediary component of the physical movement of body parts, although they also raise the possibility of the erasure of the discreteness of brain and machine. Implementations of BCIs range from non-invasive and partially invasive to invasive, based on how close electrodes get to brain tissue.
Neurotechnology encompasses any method or electronic device which interfaces with the nervous system to monitor or modulate neural activity.
Eloquent cortex is a name used by neurologists for areas of cortex that—if removed—will result in loss of sensory processing or linguistic ability, or paralysis. The most common areas of eloquent cortex are in the left temporal and frontal lobes for speech and language, bilateral occipital lobes for vision, bilateral parietal lobes for sensation, and bilateral motor cortex for movement.
Intraoperative neurophysiological monitoring (IONM) or intraoperative neuromonitoring is the use of electrophysiological methods such as electroencephalography (EEG), electromyography (EMG), and evoked potentials to monitor the functional integrity of certain neural structures during surgery. The purpose of IONM is to reduce the risk to the patient of iatrogenic damage to the nervous system, and/or to provide functional guidance to the surgeon and anesthesiologist.
Long-term or "continuous" video-electroencephalography (EEG) monitoring is a diagnostic technique commonly used in patients with epilepsy. It involves the long-term hospitalization of the patient, typically for days or weeks, during which brain waves are recorded via EEG and physical actions are continuously monitored by video. In epileptic patients, this technique is typically used to capture brain activity during seizures. The information gathered can be used for initial prognosis or long-term care management.
Frontal lobe epilepsy (FLE) is a neurological disorder that is characterized by brief, recurring seizures arising in the frontal lobes of the brain, that often occur during sleep. It is the second most common type of epilepsy after temporal lobe epilepsy (TLE), and is related to the temporal form in that both forms are characterized by partial (focal) seizures.

The sensorimotor mu rhythm, also known as mu wave, comb or wicket rhythms or arciform rhythms, are synchronized patterns of electrical activity involving large numbers of neurons, probably of the pyramidal type, in the part of the brain that controls voluntary movement. These patterns as measured by electroencephalography (EEG), magnetoencephalography (MEG), or electrocorticography (ECoG), repeat at a frequency of 7.5–12.5 Hz, and are most prominent when the body is physically at rest. Unlike the alpha wave, which occurs at a similar frequency over the resting visual cortex at the back of the scalp, the mu rhythm is found over the motor cortex, in a band approximately from ear to ear. People suppress mu rhythms when they perform motor actions or, with practice, when they visualize performing motor actions. This suppression is called desynchronization of the wave because EEG wave forms are caused by large numbers of neurons firing in synchrony. The mu rhythm is even suppressed when one observes another person performing a motor action or an abstract motion with biological characteristics. Researchers such as V. S. Ramachandran and colleagues have suggested that this is a sign that the mirror neuron system is involved in mu rhythm suppression, although others disagree.
Stereoelectroencephalography (SEEG) is the practice of recording electroencephalographic signals via depth electrodes. It may be used in patients with epilepsy not responding to medical treatment, and who are potential candidates to receive brain surgery in order to control seizures.
Epilepsy surgery involves a neurosurgical procedure where an area of the brain involved in seizures is either resected, ablated, disconnected or stimulated. The goal is to eliminate seizures or significantly reduce seizure burden. Approximately 60% of all people with epilepsy have focal epilepsy syndromes. In 15% to 20% of these patients, the condition is not adequately controlled with anticonvulsive drugs. Such patients are potential candidates for surgical epilepsy treatment.

Electroencephalography (EEG) is a method to record an electrogram of the spontaneous electrical activity of the brain. The biosignals detected by EEG have been shown to represent the postsynaptic potentials of pyramidal neurons in the neocortex and allocortex. It is typically non-invasive, with the EEG electrodes placed along the scalp using the International 10–20 system, or variations of it. Electrocorticography, involving surgical placement of electrodes, is sometimes called "intracranial EEG". Clinical interpretation of EEG recordings is most often performed by visual inspection of the tracing or quantitative EEG analysis.

Epilepsy is a neurological condition of recurrent episodes of unprovoked epileptic seizures. A seizure is an abnormal neuronal brain activity that can cause intellectual, emotional, and social consequences. Epilepsy affects children and adults of all ages and races, and is one of the most common neurological disorders of the nervous system. Epilepsy is more common among children than adults, affecting about 6 out of 1000 US children that are between the age of 0 to 5 years old. The epileptic seizures can be of different types depending on the part of the brain that was affected, seizures are classified in 2 main types partial seizure or generalized seizure.
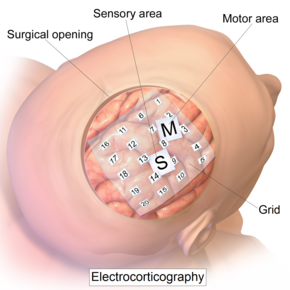
Cortical stimulation mapping (CSM) is a type of electrocorticography that involves a physically invasive procedure and aims to localize the function of specific brain regions through direct electrical stimulation of the cerebral cortex. It remains one of the earliest methods of analyzing the brain and has allowed researchers to study the relationship between cortical structure and systemic function. Cortical stimulation mapping is used for a number of clinical and therapeutic applications, and remains the preferred method for the pre-surgical mapping of the motor cortex and language areas to prevent unnecessary functional damage. There are also some clinical applications for cortical stimulation mapping, such as the treatment of epilepsy.
Awake craniotomy is a neurosurgical technique and type of craniotomy that allows a surgeon to remove a brain tumor while the patient is awake to avoid brain damage. During the surgery, the neurosurgeon performs cortical mapping to identify vital areas, called the "eloquent brain", that should not be disturbed while removing the tumor.
A cortical implant is a subset of neuroprosthetics that is in direct connection with the cerebral cortex of the brain. By directly interfacing with different regions of the cortex, the cortical implant can provide stimulation to an immediate area and provide different benefits, depending on its design and placement. A typical cortical implant is an implantable microelectrode array, which is a small device through which a neural signal can be received or transmitted.
Drug-resistant epilepsy (DRE), also known as refractory epilepsy, intractable epilepsy, or pharmacoresistant epilepsy, is diagnosed following a failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drugs (AEDs) to achieve sustained seizure freedom. The probability that the next medication will achieve seizure freedom drops with every failed AED. For example, after two failed AEDs, the probability that the third will achieve seizure freedom is around 4%. Drug-resistant epilepsy is commonly diagnosed after several years of uncontrolled seizures, however, in most cases, it is evident much earlier. Approximately 30% of people with epilepsy have a drug-resistant form.

Hal Blumenfeld is a Professor of Neurology, Neuroscience, and Neurosurgery at Yale University. He is an expert on brain mechanisms of consciousness and on altered consciousness in epilepsy. As director of the Yale Clinical Neuroscience Imaging Center (CNIC) he leads multi-disciplinary research and is also well known for his teaching contributions in neuroanatomy and clinical neuroscience.

Fabrice Bartolomei is a French neurophysiologist, and University Professor at Aix-Marseille University (AMU), leading the Service de Neurophysiologie Clinique of the Timone Hospital at the Assistance Publique - Hôpitaux de Marseille, and he is the medical director of the ‘Centre Saint-Paul - Hopital Henri Gastaut’. He is the coordinator of the clinical network CINAPSE that is dedicated to the management of adult and pediatric cases of severe epilepsy and leader of the Federation Hospitalo-Universitaire Epinext. He is also member of the research unit Institut de Neurosciences des Systèmes.
Musicogenic seizure, also known as music-induced seizure, is a rare type of seizure, with an estimated prevalence of 1 in 10,000,000 individuals, that arises from disorganized or abnormal brain electrical activity when a person hears or is exposed to a specific type of sound or musical stimuli. There are challenges when diagnosing a music-induced seizure due to the broad scope of triggers, and time delay between a stimulus and seizure. In addition, the causes of musicogenic seizures are not well-established as solely limited cases and research have been discovered and conducted respectively. Nevertheless, the current understanding of the mechanism behind musicogenic seizure is that music triggers the part of the brain that is responsible for evoking an emotion associated with that music. Dysfunction in this system leads to an abnormal release of dopamine, eventually inducing seizure.