Carbon dioxide is a chemical compound with the chemical formula CO2. It is made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature, and as the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate, which causes ocean acidification as atmospheric CO2 levels increase.

Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.

MEMS is the technology of microscopic devices incorporating both electronic and moving parts. MEMS are made up of components between 1 and 100 micrometres in size, and MEMS devices generally range in size from 20 micrometres to a millimetre, although components arranged in arrays can be more than 1000 mm2. They usually consist of a central unit that processes data and several components that interact with the surroundings.

Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon (Si) element is a significant element that is essential for several physiological and metabolic processes in plants. Si is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating.

Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is abundant as it comprises several minerals and synthetic products. All forms are white or colorless, although impure samples can be colored.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.

Silicon carbide (SiC), also known as carborundum, is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.
In semiconductor technology, copper interconnects are interconnects made of copper. They are used in silicon integrated circuits (ICs) to reduce propagation delays and power consumption. Since copper is a better conductor than aluminium, ICs using copper for their interconnects can have interconnects with narrower dimensions, and use less energy to pass electricity through them. Together, these effects lead to ICs with better performance. They were first introduced by IBM, with assistance from Motorola, in 1997.

Epitaxy refers to a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epitaxial film or epitaxial layer. The relative orientation(s) of the epitaxial layer to the seed layer is defined in terms of the orientation of the crystal lattice of each material. For most epitaxial growths, the new layer is usually crystalline and each crystallographic domain of the overlayer must have a well-defined orientation relative to the substrate crystal structure. Epitaxy can involve single-crystal structures, although grain-to-grain epitaxy has been observed in granular films. For most technological applications, single-domain epitaxy, which is the growth of an overlayer crystal with one well-defined orientation with respect to the substrate crystal, is preferred. Epitaxy can also play an important role while growing superlattice structures.

Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. SF
6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.

In semiconductor production, doping is the intentional introduction of impurities into an intrinsic (undoped) semiconductor for the purpose of modulating its electrical, optical and structural properties. The doped material is referred to as an extrinsic semiconductor.

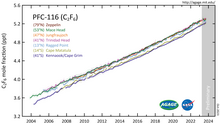
Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (CF4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond.

Nitrogen trifluoride is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
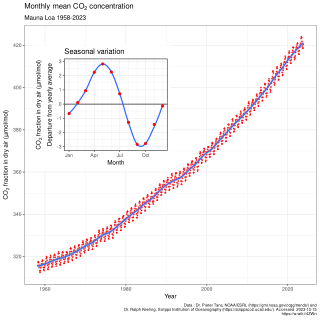
In Earth's atmosphere, carbon dioxide is a trace gas that plays an integral part in the greenhouse effect, carbon cycle, photosynthesis and oceanic carbon cycle. It is one of several greenhouse gases in the atmosphere of Earth. The current global average concentration of carbon dioxide (CO2) in the atmosphere is 421 ppm as of May 2022 (0.04%). This is an increase of 50% since the start of the Industrial Revolution, up from 280 ppm during the 10,000 years prior to the mid-18th century. The increase is due to human activity.
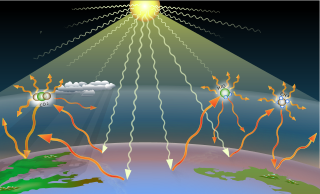
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).