Related Research Articles
Janus kinase (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-STAT pathway. They were initially named "just another kinase" 1 and 2, but were ultimately published as "Janus kinase". The name is taken from the two-faced Roman god of beginnings, endings and duality, Janus, because the JAKs possess two near-identical phosphate-transferring domains. One domain exhibits the kinase activity, while the other negatively regulates the kinase activity of the first.
The JAK-STAT signaling pathway is a chain of interactions between proteins in a cell, and is involved in processes such as immunity, cell division, cell death, and tumour formation. The pathway communicates information from chemical signals outside of a cell to the cell nucleus, resulting in the activation of genes through the process of transcription. There are three key parts of JAK-STAT signalling: Janus kinases (JAKs), signal transducer and activator of transcription proteins (STATs), and receptors. Disrupted JAK-STAT signalling may lead to a variety of diseases, such as skin conditions, cancers, and disorders affecting the immune system.

Non-receptor tyrosine-protein kinase TYK2 is an enzyme that in humans is encoded by the TYK2 gene.

Tyrosine-protein kinase JAK3 is a tyrosine kinase enzyme that in humans is encoded by the JAK3 gene.

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis. It is a janus kinase (JAK) inhibitor, discovered and developed by the National Institutes of Health and Pfizer.

Ruxolitinib, sold under the brand name Jakafi among others, is a medication used for the treatment of intermediate or high-risk myelofibrosis, a type of myeloproliferative neoplasm that affects the bone marrow; polycythemia vera, when there has been an inadequate response to or intolerance of hydroxyurea; and steroid-refractory acute graft-versus-host disease. Ruxolitinib is a Janus kinase inhibitor. It was developed and marketed by Incyte Corp in the US under the brand name Jakafi, and by Novartis elsewhere in the world, under the brand name Jakavi.
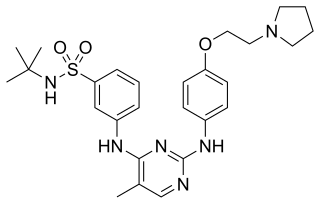
Fedratinib, sold under the brand name Inrebic, is an anti-cancer medication used to treat myeloproliferative diseases including myelofibrosis. It is used in the form of fedratinib hydrochloride capsules that are taken by mouth. It is a semi-selective inhibitor of Janus kinase 2 (JAK-2). It was approved by the FDA on 16 August 2019.

Momelotinib, sold under the brand name Ojjaara, is an anticancer medication used for the treatment of myelofibrosis. It is a Janus kinase inhibitor and it is taken by mouth.

Baricitinib, sold under the brand name Olumiant among others, is an immunomodulatory medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19. It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2.

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.

Oclacitinib, sold under the brand name Apoquel among others, is a veterinary medication used in the control of atopic dermatitis and pruritus from allergic dermatitis in dogs at least 12 months of age. Chemically, it is a synthetic cyclohexylamino pyrrolopyrimidine janus kinase inhibitor that is relatively selective for JAK1. It inhibits signal transduction when the JAK is activated and thus helps downregulate expression of inflammatory cytokines.

Upadacitinib, sold under the brand name Rinvoq, is a medication used for the treatment of rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, Crohn's disease, ankylosing spondylitis, and axial spondyloarthritis. Upadacitinib is a Janus kinase (JAK) inhibitor that works by blocking the action of enzymes called Janus kinases. These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.

Janus kinase 3 inhibitors, also called JAK3 inhibitors, are a new class of immunomodulatory agents that inhibit Janus kinase 3. They are used for the treatment of autoimmune diseases. The Janus kinases are a family of four nonreceptor tyrosine-protein kinases, JAK1, JAK2, JAK3, and TYK2. They signal via the JAK/STAT pathway, which is important in regulating the immune system. Expression of JAK3 is largely restricted to lymphocytes, while the others are ubiquitously expressed, so selective targeting of JAK3 over the other JAK isozymes is attractive as a possible treatment of autoimmune diseases.

Decernotinib is an inhibitor of Janus kinase 3 (JAK3) discovered through a process of inhouse screening of a chemical compound library. Decernotinib also had the name VX-509 in development phase. It is an experimental drug with high selectivity for JAK3, which demonstrates good efficacy in vivo in the rat host versus graft model (HvG). It has been studied in clinical trials at Vertex Pharmaceuticals, and while it was not approved for clinical use it continues to be used for research.

Cerdulatinib is a small molecule SYK/JAK kinase inhibitor in development for treatment of hematological malignancies. It has lowest nM IC50 values against TYK2, JAK1, JAK2, JAK3, FMS, and SYK.

Abrocitinib, sold under the brand name Cibinqo, is a medication used for the treatment of atopic dermatitis (eczema). It is a Janus kinase inhibitor and it was developed by Pfizer. It is taken by mouth.

An antiarthritic is any drug used to relieve or prevent arthritic symptoms, such as joint pain or joint stiffness. Depending on the antiarthritic drug class, it is used for managing pain, reducing inflammation or acting as an immunosuppressant. These drugs are typically given orally, topically or through administration by injection. The choice of antiarthritic medication is often determined by the nature of arthritis, the severity of symptoms as well as other factors, such as the tolerability of side effects.

Z583 (GLXC-26150) is a chemical compound which acts as a potent and highly selective inhibitor of JAK3, and was developed for the treatment of rheumatoid arthritis.
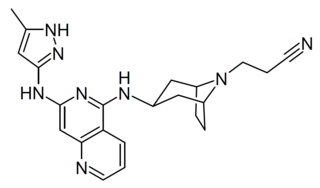
Izencitinib (TD-1473) is a drug which acts as a pan-Janus kinase inhibitor, binding with high affinity at all three subtypes JAK1, JAK2 and JAK3. It is taken orally and was developed to be gut selective with minimal absorption into the rest of the body, allowing targeting of inflammatory bowel disease but with reduced side effects compared to other similar drugs.

Gusacitinib (ASN002) is an investigational drug which acts as a pan-Janus kinase inhibitor, binding with similar affinity at JAK1, JAK2, JAK3 and TYK2, and also inhibiting spleen tyrosine kinase (SYK). It is taken orally and was developed for the treatment of eczema and dermatitis.
References
- 1 2 3 4 Kontzias A, Kotlyar A, Laurence A, Changelian P, O'Shea JJ (August 2012). "Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease". Current Opinion in Pharmacology. 12 (4): 464–70. doi:10.1016/j.coph.2012.06.008. PMC 3419278 . PMID 22819198.
- ↑ Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O'Shea JJ (June 2008). "Therapeutic targeting of Janus kinases". Immunological Reviews. 223: 132–42. doi:10.1111/j.1600-065X.2008.00644.x. PMC 2634846 . PMID 18613833.
- ↑ Norman P (August 2014). "Selective JAK inhibitors in development for rheumatoid arthritis". Expert Opinion on Investigational Drugs. 23 (8): 1067–77. doi:10.1517/13543784.2014.918604. PMID 24818516. S2CID 21143324.
- 1 2 "JAK Inhibitors Showing Promise for Many Skin Problems - Conditions ranging from alopecia to vitiligo". 6 July 2017. Archived from the original on 13 July 2017. Retrieved 9 July 2017.
- ↑ Tanaka Y, Luo Y, O'Shea JJ, Nakayamada S (5 January 2022). "Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach". Nature Reviews. Retrieved 9 March 2024.
- ↑ "JAK3-deficient severe combined immunodeficiency". Medline Plus. 1 August 2017. Retrieved 6 May 2024.
- ↑ "Janus Kinase (JAK) inhibitors: Drug Safety Communication - FDA Requires Warnings about Increased Risk of Serious Heart-related Events, Cancer, Blood Clots, and Death". U.S. Food and Drug Administration (FDA). 2 September 2021. Archived from the original on 28 October 2022. Retrieved 28 October 2022.
- ↑ "FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions". U.S. Food and Drug Administration (FDA). 6 December 2021. Archived from the original on 28 October 2022. Retrieved 28 October 2022.
- 1 2 "EMA recommends measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders". European Medicines Agency (EMA) (Press release). 28 October 2022. Retrieved 28 October 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Janus Kinase inhibitors (JAKi)". European Medicines Agency (EMA). 28 October 2022. Archived from the original on 28 October 2022. Retrieved 28 October 2022.
- ↑ Kragstrup TW, Glintborg B, Svensson AL, McMaster C, Robinson PC, Deleuran B, et al. (2022). "Waiting for JAK inhibitor safety data". RMD Open. 8 (1): e002236. doi:10.1136/rmdopen-2022-002236. PMC 8867353 . PMID 35197363.
- ↑ Furumoto Y, Gadina M (October 2013). "The arrival of JAK inhibitors: advancing the treatment of immune and hematologic disorders". BioDrugs. 27 (5): 431–8. doi:10.1007/s40259-013-0040-7. PMC 3778139 . PMID 23743669.
- ↑ "JAK inhibitors: What your dermatologist wants you to know". www.aad.org. Retrieved 30 July 2023.
- ↑ Kyoung Kim M, Shin H, Kwang-su P, Kim H, Park J, Kim K, et al. (2015). "Benzimidazole Derivatives as Potent JAK1-Selective Inhibitors". Journal of Medicinal Chemistry. 58 (18): 7596–7602. doi:10.1021/acs.jmedchem.5b01263. PMID 26351728.
- ↑ Clark JD, Flanagan ME, Telliez JB (June 2014). "Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases". Journal of Medicinal Chemistry. 57 (12): 5023–5038. doi:10.1021/jm401490p. PMID 24417533.
- ↑ Tan L, Akahane K, McNally R, Reyskens KM, Ficarro SB, Liu S, et al. (August 2015). "Development of Selective Covalent Janus Kinase 3 Inhibitors". Journal of Medicinal Chemistry. 58 (16): 6589–6606. doi:10.1021/acs.jmedchem.5b00710. PMC 4777322 . PMID 26258521.
- ↑ "Synthesis and Evaluation of 1 H -Pyrrolo[2,3- b ]pyridine Derivatives as Novel Immunomodulators Targeting Janus Kinase 3 (PDF Download Available)". ResearchGate. Retrieved 26 September 2017.
- ↑ Vaddi K, Sarlis NJ, Gupta V (November 2012). "Ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis". Expert Opinion on Pharmacotherapy. 13 (16): 2397–407. doi:10.1517/14656566.2012.732998. PMID 23051187. S2CID 29293800.
- ↑ "Jakafi (ruxolitinib) Tablets, for Oral Use. Full Prescribing Information" (PDF). Incyte Corporation. Archived (PDF) from the original on 2 April 2016. Retrieved 16 July 2016.
- ↑ Zerbini CA, Lomonte AB (May 2012). "Tofacitinib for the treatment of rheumatoid arthritis". Expert Review of Clinical Immunology. 8 (4): 319–31. doi:10.1586/eci.12.19. PMID 22607178. S2CID 12226975.
- ↑ "Xeljanz (tofacitinib) Tablets, for Oral Use and Xeljanz XR (tofacitinib) Extended Release Tablets, for Oral Use. Full Prescribing Information". Pfizer Labs. Division of Pfizer, Inc. NY, NY 10017. Archived from the original on 14 April 2019. Retrieved 16 July 2016.
- ↑ Gonzales AJ, Bowman JW, Fici GJ, Zhang M, Mann DW, Mitton-Fry M (August 2014). "Oclacitinib (Apoquel) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy". Journal of Veterinary Pharmacology and Therapeutics. 37 (4): 317–24. doi:10.1111/jvp.12101. PMC 4265276 . PMID 24495176.
- ↑ "FDA Approves Apoquel (oclacitinib tablet) to Control Itch and Inflammation in Allergic Dogs" (Press release). Zoetis. 16 May 2013. Archived from the original on 23 February 2017. Retrieved 23 February 2017.
- ↑ "Apoquel- oclacitinib maleate tablet, coated". DailyMed. 28 July 2021. Retrieved 25 June 2023.
- ↑ "FDA Approves Olumiant (baricitinib) 2-mg Tablets for the Treatment of Adults with Moderately-to-Severely Active Rheumatoid Arthritis". Eli Lilly and Company . 1 June 2018. Archived from the original on 21 August 2018. Retrieved 21 August 2018.
- ↑ "FDA Approves First Systemic Treatment for Alopecia Areata". U.S. Food and Drug Administration (FDA) (Press release). 13 June 2022. Retrieved 10 August 2022.
- ↑ Kivitz AJ, Gutierrez-Ureña SR, Poiley J, Genovese MC, Kristy R, Shay K, et al. (April 2017). "Peficitinib, a JAK Inhibitor, in the Treatment of Moderate-to-Severe Rheumatoid Arthritis in Patients With an Inadequate Response to Methotrexate". Arthritis & Rheumatology. 69 (4): 709–719. doi: 10.1002/art.39955 . PMID 27748083.
- ↑ Genovese MC, Greenwald M, Codding C, Zubrzycka-Sienkiewicz A, Kivitz AJ, Wang A, et al. (May 2017). "Peficitinib, a JAK Inhibitor, in Combination With Limited Conventional Synthetic Disease-Modifying Antirheumatic Drugs in the Treatment of Moderate-to-Severe Rheumatoid Arthritis". Arthritis & Rheumatology. 69 (5): 932–942. doi: 10.1002/art.40054 . PMID 28118538.
- ↑ Markham A, Keam SJ (June 2019). "Peficitinib: First Global Approval". Drugs. 79 (8): 887–891. doi:10.1007/s40265-019-01131-y. PMID 31093950. S2CID 155093525.
- ↑ "AbbVie Receives FDA Approval of Rinvoq (upadacitinib), an Oral JAK Inhibitor For The Treatment of Moderate to Severe Rheumatoid Arthritis". AbbVie (Press release). Retrieved 16 August 2019.
- ↑ "FDA approves treatment for patients with rare bone marrow disorder". U.S. Food and Drug Administration (FDA) (Press release). 16 August 2019. Retrieved 16 August 2019.
- ↑ "U.S. FDA Approves Inrebic (Fedratinib) as First New Treatment in Nearly a Decade for Patients With Myelofibrosis" (Press release). Celgene. 16 August 2019. Retrieved 25 June 2023– via Business Wire.
- ↑ Dhillon S (April 2020). "Delgocitinib: First Approval". Drugs. 80 (6): 609–615. doi:10.1007/s40265-020-01291-2. PMID 32166597. S2CID 212681247.
- ↑ "Clinical Trials with GLPG0634". ClinicalTrials.gov. Archived from the original on 18 October 2016. Retrieved 16 July 2016.
- ↑ "Clinical Trials with PF04965842". ClinicalTrials.gov. Archived from the original on 31 August 2021. Retrieved 21 May 2017.
- ↑ "Abrocitinib (PF-04965842)". MedChemExpress. Archived from the original on 19 March 2020. Retrieved 21 November 2019.
- ↑ "Study to Evaluate Efficacy and Safety of PF-04965842 in Subjects Aged 12 Years And Older With Moderate to Severe Atopic Dermatitis (JADE Mono-1)". ClinicalTrials.gov. 20 November 2019. Archived from the original on 22 February 2020. Retrieved 21 November 2019.
- ↑ "Opzelura (ruxolitinib) Cream, for Topical Use. Full Prescribing Information" (PDF). Incyte Corporation. Archived (PDF) from the original on 28 August 2022. Retrieved 10 September 2022.
- ↑ "Vonjo (pacritinib) Capsules, for Oral Use. Full Prescribing Information" (PDF). CTI BioPharma Corp. Archived (PDF) from the original on 10 September 2022. Retrieved 10 September 2022.
- ↑ "Sotyktu (deucravacitinib) Tablets, for Oral Use. Full Prescribing Information" (PDF). Bristol-Myers Squibb Company. Archived (PDF) from the original on 10 September 2022. Retrieved 10 September 2022.
- ↑ "Litfulo (ritlecitinib) Capsules, for Oral Use. Full Prescribing Information". Pfizer, Inc.
- ↑ "Ojjaara (momelotinib) Tablets, for Oral Use. Full Prescribing Information". DailyMed. 15 September 2023. Retrieved 20 September 2023.
- ↑ Loo WJ, Turchin I, Prajapati VH, Gooderham MJ, Grewal P, Hong CH, et al. (2023). "Clinical Implications of Targeting the JAK-STAT Pathway in Psoriatic Disease: Emphasis on the TYK2 Pathway". Journal of Cutaneous Medicine and Surgery. 27 (1_suppl): 3S–24S. doi:10.1177/12034754221141680. PMID 36519621.
- ↑ Liu D, Mamorska-Dyga A (July 2017). "Syk inhibitors in clinical development for hematological malignancies". Journal of Hematology & Oncology. 10 (1): 145. doi: 10.1186/s13045-017-0512-1 . PMC 5534090 . PMID 28754125.
- ↑ Rocha CM, Alves AM, Bettanin BF, Majolo F, Gehringer M, Laufer S, Goettert MI. Current jakinibs for the treatment of rheumatoid arthritis: a systematic review. Inflammopharmacology. 2021 Jun;29(3):595-615. doi : 10.1007/s10787-021-00822-x PMID 34046798
- ↑ Hardwick RN, Brassil P, Badagnani I, Perkins K, Obedencio GP, Kim AS, Conner MW, Bourdet DL, Harstad EB. Gut-Selective Design of Orally Administered Izencitinib (TD-1473) Limits Systemic Exposure and Effects of Janus Kinase Inhibition in Nonclinical Species. Toxicol Sci. 2022 Mar 28;186(2):323-337. doi : 10.1093/toxsci/kfac002 Error: Bad DOI specified!
- ↑ "Clinical trials with LY2784544 (Gandotinib)". ClinicalTrials.gov. Archived from the original on 6 August 2016. Retrieved 16 July 2016.
- ↑ Jimenez PA, Sofen HL, Bissonnette R, Lee M, Fowler J, Zammit DJ, et al. (August 2023). "Oral spleen tyrosine kinase/Janus Kinase inhibitor gusacitinib for the treatment of chronic hand eczema: Results of a randomized phase 2 study". Journal of the American Academy of Dermatology. 89 (2): 235–242. doi:10.1016/j.jaad.2023.04.027. PMID 37094653.
- ↑ Shabbir M, Stuart R (March 2010). "Lestaurtinib, a multitargeted tyrosine kinase inhibitor: from bench to bedside". Expert Opinion on Investigational Drugs. 19 (3): 427–36. doi:10.1517/13543781003598862. PMID 20141349. S2CID 13558158.
- ↑ Tehlirian C, Singh RS, Pradhan V, Roberts ES, Tarabar S, Peeva E, et al. (August 2022). "Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study". Journal of the American Academy of Dermatology. 87 (2): 333–342. doi:10.1016/j.jaad.2022.03.059. PMID 35398218.
- ↑ Leit S, Greenwood J, Carriero S, Mondal S, Abel R, Ashwell M, et al. (August 2023). "Discovery of a Potent and Selective Tyrosine Kinase 2 Inhibitor: TAK-279". Journal of Medicinal Chemistry. 66 (15): 10473–10496. doi:10.1021/acs.jmedchem.3c00600. PMID 37427891.
- ↑ Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM (March 2003). "Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice". Cancer Research. 63 (6): 1270–9. PMID 12649187. Archived from the original on 17 September 2016. Retrieved 16 July 2016.
- ↑ Meyer SC, Keller MD, Chiu S, Koppikar P, Guryanova OA, Rapaport F, et al. (July 2015). "CHZ868, a Type II JAK2 Inhibitor, Reverses Type I JAK Inhibitor Persistence and Demonstrates Efficacy in Myeloproliferative Neoplasms". Cancer Cell. 28 (1): 15–28. doi:10.1016/j.ccell.2015.06.006. PMC 4503933 . PMID 26175413.
- ↑ Stallard J (23 July 2015). "Discovery Could Boost New Therapies for Myeloproliferative Neoplasms". Memorial Sloan Kettering Cancer Center. Archived from the original on 16 June 2016. Retrieved 16 July 2016.
- ↑ Gershon E (19 June 2014). "In Hairless Man, Arthritis Drug Spurs Hair Growth — Lots of It". Yale News. Archived from the original on 19 July 2016. Retrieved 16 July 2016.
- ↑ Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. (October 2015). "Pharmacologic inhibition of JAK-STAT signaling promotes hair growth". Science Advances. 1 (9): e1500973. Bibcode:2015SciA....1E0973H. doi:10.1126/sciadv.1500973. PMC 4646834 . PMID 26601320.
- ↑ Kavanagh ME, Horning BD, Khattri R, et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. Nat Chem Biol 2022; 18: 1388–1398. doi : 10.1038/s41589-022-01098-0
- ↑ Chengjuan C, et al. (19 August 2022). "A highly selective JAK3 inhibitor is developed for treating rheumatoid arthritis by suppressing γc cytokine-related JAK-STAT signal". PubMed. Retrieved 6 May 2024.