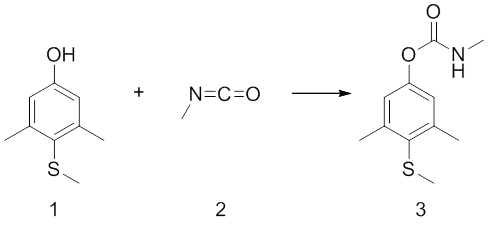
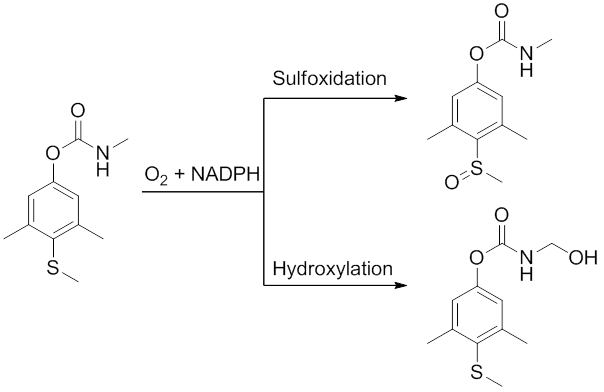
Carbaryl is a chemical in the carbamate family used chiefly as an insecticide. It is a white crystalline solid previously sold under the brand name Sevin, which was a trademark of the Bayer Company. The Sevin trademark has since been acquired by GardenTech, which has eliminated carbaryl from most Sevin formulations. Union Carbide discovered carbaryl and introduced it commercially in 1958. Bayer purchased Aventis CropScience in 2002, a company that included Union Carbide pesticide operations. Carbaryl was the third-most-used insecticide in the United States for home gardens, commercial agriculture, and forestry and rangeland protection. As a veterinary drug, it is known as carbaril (INN).

Chlorfenvinphos is an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was cancelled in 1991.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. Ethion is known to affect a neural enzyme called acetylcholinesterase and prevent it from working.
Demeton-S-methyl is an organic compound with the molecular formula C6H15O3PS2. It was used as an organothiophosphate acaricide and organothiophosphate insecticide. It is flammable. With prolonged storage, Demeton-S-methyl becomes more toxic due to formation of a sulfonium derivative which has greater affinity to the human form of the acetylcholinesterase enzyme, and this may present a hazard in agricultural use.

Aldicarb is a carbamate insecticide which is the active substance in the pesticide Temik. It is effective against thrips, aphids, spider mites, lygus, fleahoppers, and leafminers, but is primarily used as a nematicide. Aldicarb is a cholinesterase inhibitor which prevents the breakdown of acetylcholine in the synapse. Aldicarb is considered "extremely hazardous" by the EPA and World Health Organization and has been banned in more than 100 countries. In case of severe poisoning, the victim dies of respiratory failure.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

Phosmet is a phthalimide-derived, non-systemic, organophosphate insecticide used on plants and animals. It is mainly used on apple trees for control of codling moth, though it is also used on a wide range of fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, and fruit flies.
Patulin is an organic compound classified as a polyketide. It is named after the fungus from which it was isolated, Penicillium patulum. It is a white powder soluble in acidic water and in organic solvents. It is a lactone that is heat-stable, so it is not destroyed by pasteurization or thermal denaturation. However, stability following fermentation is lessened. It is a mycotoxin produced by a variety of molds, in particular, Aspergillus and Penicillium and Byssochlamys. Most commonly found in rotting apples, the amount of patulin in apple products is generally viewed as a measure of the quality of the apples used in production. In addition, patulin has been found in other foods such as grains, fruits, and vegetables. Its presence is highly regulated.

Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity.

Demeton, sold as an amber oily liquid with a sulphur like odour under the name Systox, is an organophosphate derivative causing irritability and shortness of breath to individuals repeatedly exposed. It was used as a phosphorothioate insecticide and acaricide and has the chemical formula C8H19O3PS2. Although it was previously used as an insecticide, it is now largely obsolete due to its relatively high toxicity to humans. Demeton consists of two components, demeton-S and demeton-O in a ratio of approximately 2:1 respectively. The chemical structure of demeton is closely related to military nerve agents such as VX and a derivative with one of the ethoxy groups replaced by methyl was investigated by both the US and Soviet chemical-weapons programs under the names V.sub.X and GD-7.

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.

Carbosulfan is an organic compound adherent to the carbamate class. At normal conditions, it is brown viscous liquid. It is not very stable; it decomposes slowly at room temperature. Its solubility in water is low but it is miscible with xylene, hexane, chloroform, dichloromethane, methanol and acetone. Carbosulfan is used as an insecticide. The European Union banned use of carbosulfan in 2007.

Sulfotep (also known as tetraethyldithiopyrophosphate and TEDP) is a pesticide commonly used in greenhouses as a fumigant. The substance is also known as Dithione, Dithiophos, and many other names. Sulfotep has the molecular formula C8H20O5P2S2 and belongs to the organophosphate class of chemicals. It has a cholinergic effect, involving depression of the cholinesterase activity of the peripheral and central nervous system of insects. The transduction of signals is disturbed at the synapses that make use of acetylcholine. Sulfotep is a mobile oil that is pale yellow-colored and smells like garlic. It is primarily used as an insecticide.

Ethoprophos (or ethoprop) is an organophosphate ester with the formula C8H19O2PS2. It is a clear yellow to colourless liquid that has a characteristic mercaptan-like odour. It is used as an insecticide and nematicide and it is an acetylcholinesterase inhibitor.
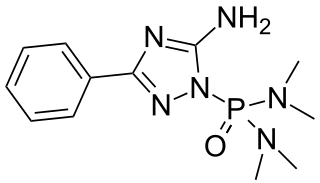
Triamiphos (chemical formula: C12H19N6OP) is an organophosphate used as a pesticide and fungicide. It is used to control powdery mildews on apples and ornamentals. It was discontinued by the US manufacturer in 1998.

Triazofos is a chemical compound used in acaricides, insecticides, and nematicides.

Ethiofencarb is a carbamate insecticide which is useful in controlling aphids on hard and soft fruits and some vegetables. It is not as dangerous as organophosphorous pesticides, but is considered highly toxic to humans in the UK, moderately toxic under US EPA classification, and highly toxic to aquatic life.

EPN is an insecticide of the phosphonothioate class. It is used against pests such as European corn borer, rice stem borer, bollworm, tobacco budworm, and boll weevil.
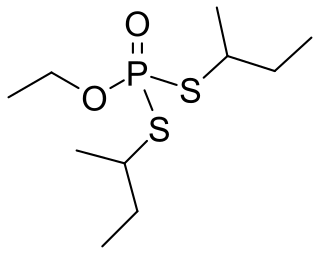
Cadusafos is a chemical insecticide and nematicide often used against parasitic nematode populations. The compound acts as a acetylcholinesterase inhibitor. It belongs the chemical class of synthetic organic thiosulfates and it is a volatile and persistent clear liquid. It is used on food crops such as tomatoes, bananas and chickpeas. It is currently not approved by the European Commission for use in the EU. Exposure can occur through inhalation, ingestion or contact with the skin. The compound is highly toxic to nematodes, earthworms and birds but poses no carcinogenic risk to humans.