Nitric oxide synthase 1 (neuronal), also known as NOS1, is an enzyme that in humans is encoded by the NOS1 gene. [5] [6]
Nitric oxide synthase 1 (neuronal), also known as NOS1, is an enzyme that in humans is encoded by the NOS1 gene. [5] [6]
Nitric oxide synthases (EC 1.14.13.39) (NOSs) are a family of synthases that catalyze the production of nitric oxide (NO) from L-arginine. NO is a chemical messenger with diverse functions throughout the body depending on its enzymatic source and tissue localization. In the brain and peripheral nervous system, where NOS1 is largely present, NO displays many properties of a neurotransmitter and may be involved in long term potentiation. It is implicated in neurotoxicity associated with stroke and neurodegenerative diseases, neural regulation of smooth muscle, including peristalsis and sphincter relaxation, and penile erection. NO is also responsible for endothelium-derived relaxing factor activity regulating blood pressure as produced from its related enzyme NOS3. In macrophages, NO mediates tumoricidal and bactericidal actions, as produced from its related enzyme NOS2. Various pharmacological inhibitors of NO synthases (NOS) block these effects, but further distinction of their function has been elucidated by animal models in which these specific genes have been inactivated. Neuronal NOS (NOS1), Endothelial NOS (NOS3), and Inducible NOS macrophage NOS are distinct isoforms. [7] Both the neuronal and the macrophage forms are unusual among oxidative enzymes in requiring several electron donors: flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), NADPH, and tetrahydrobiopterin. [8]
It has been implicated in asthma, [9] [10] schizophrenia, [11] [12] restless leg syndrome, [13] and psychostimulant neurotoxicity. It has also been investigated with respect to bipolar disorder [14] and air pollution exposure. [15]
NOS1 has been shown to interact with DLG4 [16] [17] and NOS1AP. [16]

Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. NO is an important cellular signaling molecule. It helps modulate vascular tone, insulin secretion, airway tone, and peristalsis, and is involved in angiogenesis and neural development. It may function as a retrograde neurotransmitter. Nitric oxide is mediated in mammals by the calcium-calmodulin controlled isoenzymes eNOS and nNOS. The inducible isoform, iNOS, involved in immune response, binds calmodulin at physiologically relevant concentrations, and produces NO as an immune defense mechanism, as NO is a free radical with an unpaired electron. It is the proximate cause of septic shock and may function in autoimmune disease.

GTP cyclohydrolase I (GTPCH) (EC 3.5.4.16) is a member of the GTP cyclohydrolase family of enzymes. GTPCH is part of the folate and biopterin biosynthesis pathways. It is responsible for the hydrolysis of guanosine triphosphate (GTP) to form 7,8-dihydroneopterin triphosphate (7,8-DHNP-3'-TP, 7,8-NH2-3'-TP).

Endothelial NOS (eNOS), also known as nitric oxide synthase 3 (NOS3) or constitutive NOS (cNOS), is an enzyme that in humans is encoded by the NOS3 gene located in the 7q35-7q36 region of chromosome 7. This enzyme is one of three isoforms that synthesize nitric oxide (NO), a small gaseous and lipophilic molecule that participates in several biological processes. The other isoforms include neuronal nitric oxide synthase (nNOS), which is constitutively expressed in specific neurons of the brain and inducible nitric oxide synthase (iNOS), whose expression is typically induced in inflammatory diseases. eNOS is primarily responsible for the generation of NO in the vascular endothelium, a monolayer of flat cells lining the interior surface of blood vessels, at the interface between circulating blood in the lumen and the remainder of the vessel wall. NO produced by eNOS in the vascular endothelium plays crucial roles in regulating vascular tone, cellular proliferation, leukocyte adhesion, and platelet aggregation. Therefore, a functional eNOS is essential for a healthy cardiovascular system.
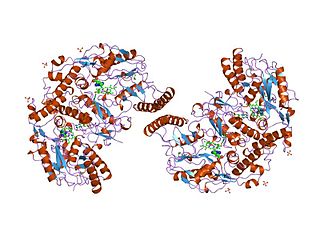
Nitric oxide synthase, inducible is an enzyme which is encoded by the NOS2 gene in humans and mice.
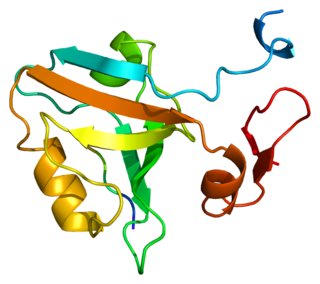
PSD-95 also known as SAP-90 is a protein that in humans is encoded by the DLG4 gene.

Calcium/calmodulin-dependent protein kinase type IV is an enzyme that in humans is encoded by the CAMK4 gene.

Disks large homolog 2 (DLG2) also known as channel-associated protein of synapse-110 (chapsyn-110) or postsynaptic density protein 93 (PSD-93) is a protein that in humans is encoded by the DLG2 gene.
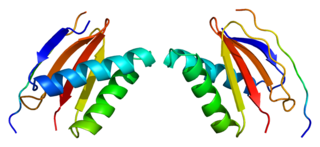
Dynein light chain 1, cytoplasmic is a protein that in humans is encoded by the DYNLL1 gene.
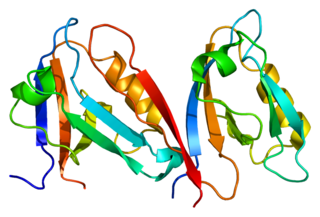
Alpha-1-syntrophin is a protein that in humans is encoded by the SNTA1 gene. Alpha-1 syntrophin is a signal transducing adaptor protein and serves as a scaffold for various signaling molecules. Alpha-1 syntrophin contains a PDZ domain, two Pleckstrin homology domain and a 'syntrophin unique' domain.

Calcium/calmodulin-dependent protein kinase type 1 is an enzyme that in humans is encoded by the CAMK1 gene.

Glutamate [NMDA] receptor subunit 3B is a protein that in humans is encoded by the GRIN3B gene.

Nitric oxide synthase 1 adaptor protein (NOS1AP) also known as carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase protein (CAPON) is a protein that in humans is encoded by the NOS1AP gene.

Brain mitochondrial carrier protein 1 is a protein that in humans is encoded by the SLC25A14 gene.

Nitric oxide synthase-interacting protein is an enzyme that in humans is encoded by the NOSIP gene.

7-Nitroindazole, or 7-NI, is a heterocyclic small molecule containing an indazole ring that has been nitrated at the 7 position. Nitroindazole acts as a selective inhibitor for neuronal nitric oxide synthase, a hemoprotein enzyme that, in neuronal tissue, converts arginine to citrulline and nitric oxide (NO). Nitric oxide can diffuse through the plasma membrane into neighbouring cells, allowing cell signalling, so nitroindazole indirectly inhibits this signalling process. Other inhibitors exist such as 3-bromo-7-nitroindazole, which is more potent but less specific, or NPA (N-propyl-L-arginine), which acts on a different site.

In medicine, exhaled nitric oxide (eNO) can be measured in a breath test for asthma and other respiratory conditions characterized by airway inflammation. Nitric oxide (NO) is a gaseous molecule produced by certain cell types in an inflammatory response. The fraction of exhaled NO (FENO) is a promising biomarker for the diagnosis, follow-up and as a guide to therapy in adults and children with asthma. The breath test has recently become available in many well-equipped hospitals in developed countries, although its exact role remains unclear.
Biological functions of nitric oxide are roles that nitric oxide plays within biology.
Flavoprotein pyridine nucleotide cytochrome reductases catalyse the interchange of reducing equivalents between one-electron carriers and the two-electron-carrying nicotinamide dinucleotides. The enzymes include ferredoxin-NADP+ reductases, plant and fungal NAD(P)H:nitrate reductases, cytochrome b5 reductases, cytochrome P450 reductases, sulphite reductases, nitric oxide synthases, phthalate dioxygenase reductase, and various other flavoproteins.
Arginase, type II is an arginase protein that in humans is encoded by the ARG2 gene.
David S. Bredt is an American molecular neuroscientist.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.