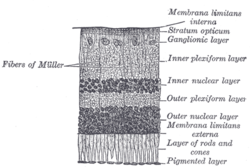
The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then processes that image within the retina and sends nerve impulses along the optic nerve to the visual cortex to create visual perception. The retina serves a function which is in many ways analogous to that of the film or image sensor in a camera.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.
In visual physiology, adaptation is the ability of the retina of the eye to adjust to various levels of light. Natural night vision, or scotopic vision, is the ability to see under low-light conditions. In humans, rod cells are exclusively responsible for night vision as cone cells are only able to function at higher illumination levels. Night vision is of lower quality than day vision because it is limited in resolution and colors cannot be discerned; only shades of gray are seen. In order for humans to transition from day to night vision they must undergo a dark adaptation period of up to two hours in which each eye adjusts from a high to a low luminescence "setting", increasing sensitivity hugely, by many orders of magnitude. This adaptation period is different between rod and cone cells and results from the regeneration of photopigments to increase retinal sensitivity. Light adaptation, in contrast, works very quickly, within seconds.

Scanning laser ophthalmoscopy (SLO) is a method of examination of the eye. It uses the technique of confocal laser scanning microscopy for diagnostic imaging of the retina or cornea of the human eye.
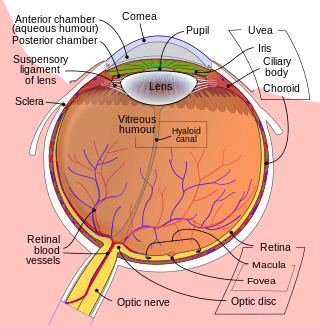
The fovea centralis is a small, central pit composed of closely packed cones in the eye. It is located in the center of the macula lutea of the retina.

Macular degeneration, also known as age-related macular degeneration, is a medical condition which may result in blurred or no vision in the center of the visual field. Early on there are often no symptoms. Over time, however, some people experience a gradual worsening of vision that may affect one or both eyes. While it does not result in complete blindness, loss of central vision can make it hard to recognize faces, drive, read, or perform other activities of daily life. Visual hallucinations may also occur.

A cone dystrophy is an inherited ocular disorder characterized by the loss of cone cells, the photoreceptors responsible for both central and color vision.

Vitelliform macular dystrophy is an irregular autosomal dominant eye disorder which can cause progressive vision loss. This disorder affects the retina, specifically cells in a small area near the center of the retina called the macula. The macula is responsible for sharp central vision, which is needed for detailed tasks such as reading, driving, and recognizing faces. The condition is characterized by yellow, slightly elevated, round structures similar to the yolk of an egg.

Epiretinal membrane or macular pucker is a disease of the eye in response to changes in the vitreous humor or more rarely, diabetes. Sometimes, as a result of immune system response to protect the retina, cells converge in the macular area as the vitreous ages and pulls away in posterior vitreous detachment (PVD).
The visual cycle is a process in the retina that replenishes the molecule retinal for its use in vision. Retinal is the chromophore of most visual opsins, meaning it captures the photons to begin the phototransduction cascade. When the photon is absorbed, the 11-cis retinal photoisomerizes into all-trans retinal as it is ejected from the opsin protein. Each molecule of retinal must travel from the photoreceptor cell to the RPE and back in order to be refreshed and combined with another opsin. This closed enzymatic pathway of 11-cis retinal is sometimes called Wald's visual cycle after George Wald (1906–1997), who received the Nobel Prize in 1967 for his work towards its discovery.

Disc shedding is the process by which photoreceptor cells in the retina are renewed. The disc formations in the outer segment of photoreceptors, which contain the photosensitive opsins, are completely renewed every ten days.

Mammals normally have a pair of eyes. Although mammalian vision is not so excellent as bird vision, it is at least dichromatic for most of mammalian species, with certain families possessing a trichromatic color perception.
White dot syndromes are inflammatory diseases characterized by the presence of white dots on the fundus, the interior surface of the eye. The majority of individuals affected with white dot syndromes are younger than fifty years of age. Some symptoms include blurred vision and visual field loss. There are many theories for the etiology of white dot syndromes including infectious, viral, genetics and autoimmune.
Proliferative vitreoretinopathy (PVR) is a disease that develops as a complication of rhegmatogenous retinal detachment. PVR occurs in about 8–10% of patients undergoing primary retinal detachment surgery and prevents the successful surgical repair of rhegmatogenous retinal detachment. PVR can be treated with surgery to reattach the detached retina but the visual outcome of the surgery is very poor. A number of studies have explored various possible adjunctive agents for the prevention and treatment of PVR, such as methotrexate, although none have yet been licensed for clinical use.
Retinal gene therapy holds a promise in treating different forms of non-inherited and inherited blindness.
Berlin's edema a common condition caused by blunt injury to the eye. It is characterized by decreased vision in the injured eye a few hours after the injury. Under examination the retina appears opaque and white in colour in the periphery but the blood vessels are normally seen along with "cherry red spot" in the foveal region. This whitening is indicative of cell damage, which occurs in the retinal pigment epithelium and outer segment layer of photoreceptors. Damage to the outer segment often results in photoreceptor death through uncertain mechanisms. Usually there is no leakage of fluid and therefore it is not considered a true edema. The choroidal fluorescence in fluorescent angiography is absent. Visual acuity ranges from 20/20 to 20/400.

Emixustat is a small molecule notable for its establishment of a new class of compounds known as visual cycle modulators (VCMs). Formulated as the hydrochloride salt, emixustat hydrochloride, it is the first synthetic medicinal compound shown to affect retinal disease processes when taken by mouth. Emixustat was invented by the British-American chemist, Ian L. Scott, and is currently in Phase 3 trials for dry, age-related macular degeneration (AMD).
Geographic atrophy (GA), also known as atrophic age-related macular degeneration (AMD) or advanced dry AMD, is an advanced form of age-related macular degeneration that can result in the progressive and irreversible loss of retinal tissue (photoreceptors, retinal pigment epithelium, choriocapillaris) which can lead to a loss of visual function over time. It is estimated that GA affects over 5 million people worldwide and approximately 1 million patients in the US, which is similar to the prevalence of neovascular (wet) AMD, the other advanced form of the disease.

Stem cell therapy for macular degeneration is the use of stem cells to heal, replace dead or damaged cells of the macula in the retina. Stem cell based therapies using bone marrow stem cells as well as retinal pigment epithelial transplantation are being studied. A number of trials have occurred in humans with encouraging results.
Congenital hypertrophy of the retinal pigment epithelium (CHRPE) is a harmless, pigmented fundus lesion that can be of various forms: solitary, grouped, and atypical, and are found through clinical eye screenings from digital retinal imaging often established by ophthalmologists. It is an uncommon diagnostic that is primarily found in patients before reaching their 30s, with the lesions often enlarging with time, thus being difficult to detect in younger ages. With the detection of CHRPE, patients with CHRPE appearing with multiple shaped lesions are often the ones known to have Familial adenomatous polyposis (FAP). Procedures are done to help establish further understanding of what is to be done when traces of CHRPE are found through eye exams, with education being a top priority for patients who have the CHRPE lesions to help them determine what the next steps are. With this discovery, patients are highly encouraged to receive a colonoscopy in order to detect colorectal polyps, these often having high risks of being cancerous.