Etymology

The ryanodine receptors are named after the plant alkaloid ryanodine which shows a high affinity to them.
| RyR domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | RyR | ||||||||
| Pfam | PF02026 | ||||||||
| InterPro | IPR003032 | ||||||||
| TCDB | 1.A.3 | ||||||||
| OPM superfamily | 8 | ||||||||
| OPM protein | 5gl0 | ||||||||
| |||||||||
Ryanodine receptors (RyR for short) form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. [1] There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.

The ryanodine receptors are named after the plant alkaloid ryanodine which shows a high affinity to them.
There are multiple isoforms of ryanodine receptors:
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ryanodine receptors mediate the release of calcium ions from the sarcoplasmic reticulum and endoplasmic reticulum, an essential step in muscle contraction. [1] In skeletal muscle, activation of ryanodine receptors occurs via a physical coupling to the dihydropyridine receptor (a voltage-dependent, L-type calcium channel), whereas, in cardiac muscle, the primary mechanism of activation is calcium-induced calcium release, which causes calcium outflow from the sarcoplasmic reticulum. [3]
It has been shown that calcium release from a number of ryanodine receptors in a ryanodine receptor cluster results in a spatiotemporally restricted rise in cytosolic calcium that can be visualised as a calcium spark . [4] Ryanodine receptors are very close to mitochondria and calcium release from RyR has been shown to regulate ATP production in heart and pancreas cells. [5] [6] [7]
Ryanodine receptors are similar to the inositol trisphosphate (IP3 or InsP3) receptor, and stimulated to transport Ca2+ into the cytosol by recognizing Ca2+ on its cytosolic side, thus establishing a positive feedback mechanism; a small amount of Ca2+ in the cytosol near the receptor will cause it to release even more Ca2+ (calcium-induced calcium release/CICR). [1] However, as the concentration of intracellular Ca2+ rises, this can trigger closing of RyR, preventing the total depletion of SR. This finding therefore indicates that a plot of opening probability for RyR as a function of Ca2+ concentration is a bell-curve. [8] Furthermore, RyR can sense the Ca2+ concentration inside the ER/SR and spontaneously open in a process known as store overload-induced calcium release (SOICR). [9]
RyRs are especially important in neurons and muscle cells. In heart and pancreas cells, another second messenger (cyclic ADP-ribose) takes part in the receptor activation.
The localized and time-limited activity of Ca2+ in the cytosol is also called a Ca2+ wave. The building of the wave is done by
RyRs form docking platforms for a multitude of proteins and small molecule ligands. [1] The cardiac-specific isoform of the receptor (RyR2) is known to form a quaternary complex with luminal calsequestrin, junctin, and triadin. [10] Calsequestrin has multiple Ca2+ binding sites and binds Ca2+ ions with very low affinity so they can be easily released.
A variety of other molecules may interact with and regulate ryanodine receptor. For example: dimerized Homer physical tether linking inositol trisphosphate receptors (IP3R) and ryanodine receptors on the intracellular calcium stores with cell surface group 1 metabotropic glutamate receptors and the Alpha-1D adrenergic receptor [14]
The plant alkaloid ryanodine, for which this receptor was named, has become an invaluable investigative tool. It can block the phasic release of calcium, but at low doses may not block the tonic cumulative calcium release. The binding of ryanodine to RyRs is use-dependent, that is the channels have to be in the activated state. At low (<10 micromolar, works even at nanomolar) concentrations, ryanodine binding locks the RyRs into a long-lived subconductance (half-open) state and eventually depletes the store, while higher (~100 micromolar) concentrations irreversibly inhibit channel-opening.
RyRs are activated by millimolar caffeine concentrations. High (greater than 5 mmol/L) caffeine concentrations cause a pronounced increase (from micromolar to picomolar) in the sensitivity of RyRs to Ca2+ in the presence of caffeine, such that basal Ca2+ concentrations become activatory. At low millimolar caffeine concentrations, the receptor opens in a quantal way, but has complicated behavior in terms of repeated use of caffeine or dependence on cytosolic or luminal calcium concentrations.
RyR1 mutations are associated with malignant hyperthermia and central core disease. [15] Mutant-type RyR1 receptors exposed to volatile anesthetics or other triggering agents can display an increased affinity for cytoplasmic Ca2+ at activating sites as well as a decreased cytoplasmic Ca2+ affinity at inhibitory sites. [16] The breakdown of this feedback mechanism causes uncontrolled release of Ca2+ into the cytoplasm, and increased ATP hydrolysis resulting from ATPase enzymes shuttling Ca2+ back into the sarcoplasmic reticulum leads to excessive heat generation. [17]
RyR2 mutations play a role in stress-induced polymorphic ventricular tachycardia (a form of cardiac arrhythmia) and ARVD. [2] It has also been shown that levels of type RyR3 are greatly increased in PC12 cells overexpressing mutant human Presenilin 1, and in brain tissue in knockin mice that express mutant Presenilin 1 at normal levels, [18] and thus may play a role in the pathogenesis of neurodegenerative diseases, like Alzheimer's disease. [19]
The presence of antibodies against ryanodine receptors in blood serum has also been associated with myasthenia gravis. [1]
Sudden cardiac death in several young individuals in the Amish community (four of which were from the same family) was traced to homozygous duplication of a mutant RyR2 (Ryanodine Receptor) gene. [20] Normal (wild type) ryanodine receptors are involved in CICR in heart and other muscles, and RyR2 functions primarily in the myocardium (heart muscle).

Ryanodine receptors are multidomain homotetramers which regulate intracellular calcium ion release from the sarcoplasmic and endoplasmic reticula. [21] They are the largest known ion channels, with weights exceeding 2 megadaltons, and their structural complexity enables a wide variety of allosteric regulation mechanisms. [22] [23]
RyR1 cryo-EM structure revealed a large cytosolic assembly built on an extended α-solenoid scaffold connecting key regulatory domains to the pore. The RyR1 pore architecture shares the general structure of the six-transmembrane ion channel superfamily. A unique domain inserted between the second and third transmembrane helices interacts intimately with paired EF-hands originating from the α-solenoid scaffold, suggesting a mechanism for channel gating by Ca2+. [1] [24]
Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.
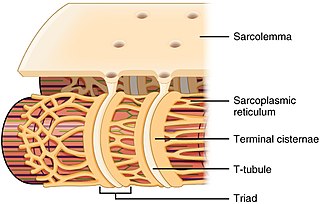
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.
Calcium-induced calcium release (CICR) describes a biological process whereby calcium is able to activate calcium release from intracellular Ca2+ stores (e.g., endoplasmic reticulum or sarcoplasmic reticulum). Although CICR was first proposed for skeletal muscle in the 1970s, it is now known that CICR is unlikely to be the primary mechanism for activating SR calcium release. Instead, CICR is thought to be crucial for excitation-contraction coupling in cardiac muscle. It is now obvious that CICR is a widely occurring cellular signaling process present even in many non-muscle cells, such as in the insulin-secreting pancreatic beta cells, epithelium, and many other cells. Since CICR is a positive-feedback system, it has been of great interest to elucidate the mechanism(s) responsible for its termination.

Ryanodine is a poisonous diterpenoid found in the South American plant Ryania speciosa (Salicaceae). It was originally used as an insecticide.

Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited genetic disorder that predisposes those affected to potentially life-threatening abnormal heart rhythms or arrhythmias. The arrhythmias seen in CPVT typically occur during exercise or at times of emotional stress, and classically take the form of bidirectional ventricular tachycardia or ventricular fibrillation. Those affected may be asymptomatic, but they may also experience blackouts or even sudden cardiac death.
A calcium spark is the microscopic release of calcium (Ca2+) from a store known as the sarcoplasmic reticulum (SR), located within muscle cells. This release occurs through an ion channel within the membrane of the SR, known as a ryanodine receptor (RyR), which opens upon activation. This process is important as it helps to maintain Ca2+ concentration within the cell. It also initiates muscle contraction in skeletal and cardiac muscles and muscle relaxation in smooth muscles. Ca2+ sparks are important in physiology as they show how Ca2+ can be used at a subcellular level, to signal both local changes, known as local control, as well as whole cell changes.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.

Triadin, also known as TRDN, is a human gene associated with the release of calcium ions from the sarcoplasmic reticulum triggering muscular contraction through calcium-induced calcium release. Triadin is a multiprotein family, arising from different processing of the TRDN gene on chromosome 6. It is a transmembrane protein on the sarcoplasmic reticulum due to a well defined hydrophobic section and it forms a quaternary complex with the cardiac ryanodine receptor (RYR2), calsequestrin (CASQ2) and junctin proteins. The luminal (inner compartment of the sarcoplasmic reticulum) section of Triadin has areas of highly charged amino acid residues that act as luminal Ca2+ receptors. Triadin is also able to sense luminal Ca2+ concentrations by mediating interactions between RYR2 and CASQ2. Triadin has several different forms; Trisk 95 and Trisk 51, which are expressed in skeletal muscle, and Trisk 32 (CT1), which is mainly expressed in cardiac muscle.

Ryanodine receptor 1 (RYR-1) also known as skeletal muscle calcium release channel or skeletal muscle-type ryanodine receptor is one of a class of ryanodine receptors and a protein found primarily in skeletal muscle. In humans, it is encoded by the RYR1 gene.
Imperatoxin I (IpTx) is a peptide toxin derived from the venom of the African scorpion Pandinus imperator.
JTV-519 (K201) is a 1,4-benzothiazepine derivative that interacts with many cellular targets. It has many structural similarities to diltiazem, a Ca2+ channel blocker used for treatment of hypertension, angina pectoris and some types of arrhythmias. JTV-519 acts in the sarcoplasmic reticulum (SR) of cardiac myocytes by binding to and stabilizing the ryanodine receptor (RyR2) in its closed state. It can be used in the treatment of cardiac arrhythmias, heart failure, catecholaminergic polymorphic ventricular tachycardia (CPVT) and store overload-induced Ca2+ release (SOICR). Currently, this drug has only been tested on animals and its side effects are still unknown. As research continues, some studies have also found a dose-dependent response; where there is no improvement seen in failing hearts at 0.3 μM and a decline in response at 1 μM.
CXL 1020 is an experimental drug that is being investigated as a treatment for acute decompensated heart failure. CXL 1020 functions as a nitroxyl donor; nitroxyl is the reduced, protonated version of nitric oxide. Nitroxyl is capable of enhancing left ventricular contractility without increasing heart rate by modifying normal Ca2+ cycling through the sarcoplasmic reticulum as well as increasing the sensitivity of cardiac myofilaments to Ca2+.

Hadrucalcin is a peptide toxin from the venom of the scorpion Hadrurus gertschi. Hadrucalcin modifies the Ryanodine receptor channels RyR1 and RyR2, found in the sarcoplasmic reticulum, to a long-lasting subconductance state, thus inducing the release of calcium from the sarcoplasmic reticulum.
The dyadic space is the name for the volume of cytoplasm between pairs (dyads) of areas where the cell membrane and an organelle such as the endoplasmic reticulum come into close contact of each other, creating what are known as dyadic clefts.
The ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channel (RIR-CaC) family includes Ryanodine receptors and Inositol trisphosphate receptors. Members of this family are large proteins, some exceeding 5000 amino acyl residues in length. This family belongs to the Voltage-gated ion channel (VIC) superfamily. Ry receptors occur primarily in muscle cell sarcoplasmic reticular (SR) membranes, and IP3 receptors occur primarily in brain cell endoplasmic reticular (ER) membranes where they effect release of Ca2+ into the cytoplasm upon activation (opening) of the channel. They are redox sensors, possibly providing a partial explanation for how they control cytoplasmic Ca2+. Ry receptors have been identified in heart mitochondria where they provide the main pathway for Ca2+ entry. Sun et al. (2011) have demonstrated oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel (RyR1;TC# 1.A.3.1.2) by NADPH oxidase 4.
Cardiac excitation-contraction coupling (CardiacEC coupling) describes the series of events, from the production of an electrical impulse (action potential) to the contraction of muscles in the heart. This process is of vital importance as it allows for the heart to beat in a controlled manner, without the need for conscious input. EC coupling results in the sequential contraction of the heart muscles that allows blood to be pumped, first to the lungs (pulmonary circulation) and then around the rest of the body (systemic circulation) at a rate between 60 and 100 beats every minute, when the body is at rest. This rate can be altered, however, by nerves that work to either increase heart rate (sympathetic nerves) or decrease it (parasympathetic nerves), as the body's oxygen demands change. Ultimately, muscle contraction revolves around a charged atom (ion), calcium (Ca2+), which is responsible for converting the electrical energy of the action potential into mechanical energy (contraction) of the muscle. This is achieved in a region of the muscle cell, called the transverse tubule during a process known as calcium induced calcium release.
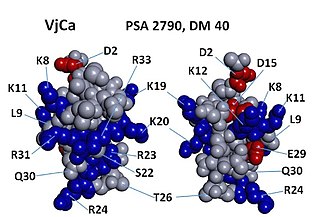
Vejocalcin (VjCa, also called Vejocalcine) is a toxin from the venom of the Mexican scorpion Vaejovis mexicanus. Vejocalcin is a member of the calcin family of toxins. It acts as a cell-penetrating peptide (CPP); it binds with high affinity and specificity to skeletal ryanodine receptor 1 (RYR1) of the sarcoplasmic reticulum, thereby triggering calcium release from intracellular Ca2+ stores.