Hydrolase is a class of enzymes that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are esterases including lipases, phosphatases, glycosidases, peptidases, and nucleosidases.
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.

Bile salt-dependent lipase, also known as carboxyl ester lipase is an enzyme produced by the adult pancreas and aids in the digestion of fats. Bile salt-stimulated lipase is an equivalent enzyme found within breast milk. BSDL has been found in the pancreatic secretions of all species in which it has been looked for. BSSL, originally discovered in the milk of humans and various other primates, has since been found in the milk of many animals including dogs, cats, rats, and rabbits.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:
The enzyme acetylsalicylate deacetylase (EC 3.1.1.55) catalyzes the reaction
The enzyme acylcarnitine hydrolase (EC 3.1.1.28) catalyzes the reaction
The enzyme α-amino-acid esterase (EC 3.1.1.43) catalyzes the reaction
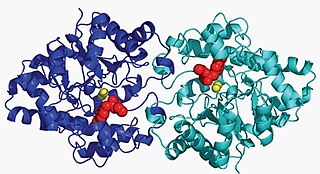
Aryldialkylphosphatase is a metalloenzyme that hydrolyzes the triester linkage found in organophosphate insecticides:
The enzyme arylesterase (EC 3.1.1.2) catalyzes the reaction
The enzyme bis(2-ethylhexyl)phthalate esterase (EC 3.1.1.60) catalyzes the reaction

The enzyme cutinase is a member of the hydrolase family. It catalyzes the following reaction:
The enzyme feruloyl esterase (EC 3.1.1.73) catalyzes the reaction
The enzyme lysophospholipase (EC 3.1.1.5) catalyzes the reaction
The enzyme S-formylglutathione hydrolase (EC 3.1.2.12) catalyzes the reaction
The enzyme sterol esterase (EC 3.1.1.13) catalyzes the reaction

Carboxylesterase, type B is a family of evolutionarily related proteins that belongs to the superfamily of proteins with the Alpha/beta hydrolase fold.
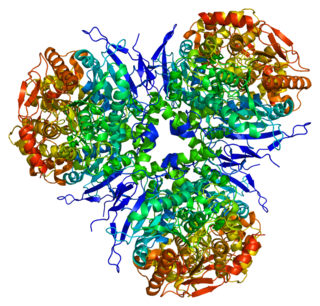
Liver carboxylesterase 1 also known as carboxylesterase 1 is an enzyme that in humans is encoded by the CES1 gene. The protein is also historically known as serine esterase 1 (SES1), monocyte esterase and cholesterol ester hydrolase (CEH). Three transcript variants encoding three different isoforms have been found for this gene. The various protein products from isoform a, b and c range in size from 568, 567 and 566 amino acids long, respectively.
The enzyme cocaine esterase (EC 3.1.1.84, CocE, hCE2, hCE-2, human carboxylesterase 2; systematic name cocaine benzoylhydrolase) catalyses the reaction
The enzyme pyrethroid hydrolase (EC 3.1.1.88, pyrethroid-hydrolyzing carboxylesterase, pyrethroid-hydrolysing esterase, pyrethroid-hydrolyzing esterase, pyrethroid-selective esterase, pyrethroid-cleaving enzyme, permethrinase, PytH, EstP; systematic name pyrethroid-ester hydrolase) catalyses the reaction

Edwin Clifford Webb (1921–2006) was a biochemist. He studied nerve gases at the University of Cambridge where he was a Beit Fellow and lecturer. He had earned his doctorate there, working in the laboratory of Malcolm Dixon and continued to collaborate with him in the study of enzymes. Together, they wrote a classic textbook on the subject, Enzymes, which was first published by Longmans in 1958, He then took a chair in biochemistry at the University of Queensland but continued to collaborate with Dixon on further editions. In 1970, he became the Deputy Vice-Chancellor at Queensland and in 1975 he became the second Vice-Chancellor of Macquarie University. He retired in 1986 but continued to work on the enzyme list of the International Union of Biochemistry and Molecular Biology (IUBMB) while living in Townsville.







