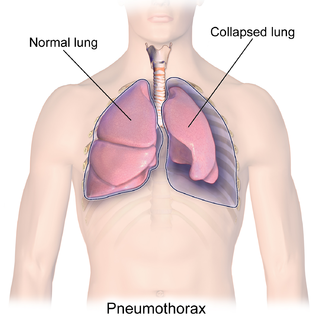
A pneumothorax is an abnormal collection of air in the pleural space between the lung and the chest wall. Symptoms typically include sudden onset of sharp, one-sided chest pain and shortness of breath. In a minority of cases, a one-way valve is formed by an area of damaged tissue, and the amount of air in the space between chest wall and lungs increases; this is called a tension pneumothorax. This can cause a steadily worsening oxygen shortage and low blood pressure. This leads to a type of shock called obstructive shock, which can be fatal unless reversed. Very rarely, both lungs may be affected by a pneumothorax. It is often called a "collapsed lung", although that term may also refer to atelectasis.

Pleurisy, also known as pleuritis, is inflammation of the membranes that surround the lungs and line the chest cavity (pleurae). This can result in a sharp chest pain while breathing. Occasionally the pain may be a constant dull ache. Other symptoms may include shortness of breath, cough, fever, or weight loss, depending on the underlying cause. Pleurisy can be caused by a variety of conditions, including viral or bacterial infections, autoimmune disorders, and pulmonary embolism.

Nasogastric intubation is a medical process involving the insertion of a plastic tube through the nose, down the esophagus, and down into the stomach. Orogastric intubation is a similar process involving the insertion of a plastic tube through the mouth. Abraham Louis Levin invented the NG tube. Nasogastric tube is also known as Ryle's tube in Commonwealth countries, after John Alfred Ryle.

A pleural effusion is accumulation of excessive fluid in the pleural space, the potential space that surrounds each lung. Under normal conditions, pleural fluid is secreted by the parietal pleural capillaries at a rate of 0.6 millilitre per kilogram weight per hour, and is cleared by lymphatic absorption leaving behind only 5–15 millilitres of fluid, which helps to maintain a functional vacuum between the parietal and visceral pleurae. Excess fluid within the pleural space can impair inspiration by upsetting the functional vacuum and hydrostatically increasing the resistance against lung expansion, resulting in a fully or partially collapsed lung.

Pleural empyema is a collection of pus in the pleural cavity caused by microorganisms, usually bacteria. Often it happens in the context of a pneumonia, injury, or chest surgery. It is one of the various kinds of pleural effusion. There are three stages: exudative, when there is an increase in pleural fluid with or without the presence of pus; fibrinopurulent, when fibrous septa form localized pus pockets; and the final organizing stage, when there is scarring of the pleura membranes with possible inability of the lung to expand. Simple pleural effusions occur in up to 40% of bacterial pneumonias. They are usually small and resolve with appropriate antibiotic therapy. If however an empyema develops additional intervention is required.

Pleurodesis is a medical procedure in which part of the pleural space is artificially obliterated. It involves the adhesion of the visceral and the costal pleura. The mediastinal pleura is spared.

A thoracotomy is a surgical procedure to gain access into the pleural space of the chest. It is performed by surgeons to gain access to the thoracic organs, most commonly the heart, the lungs, or the esophagus, or for access to the thoracic aorta or the anterior spine. A thoracotomy is the first step in thoracic surgeries including lobectomy or pneumonectomy for lung cancer or to gain thoracic access in major trauma.

A hemothorax is an accumulation of blood within the pleural cavity. The symptoms of a hemothorax may include chest pain and difficulty breathing, while the clinical signs may include reduced breath sounds on the affected side and a rapid heart rate. Hemothoraces are usually caused by an injury, but they may occur spontaneously due to cancer invading the pleural cavity, as a result of a blood clotting disorder, as an unusual manifestation of endometriosis, in response to pneumothorax, or rarely in association with other conditions.

A chylothorax is an abnormal accumulation of chyle, a type of lipid-rich lymph, in the space surrounding the lung. The lymphatics of the digestive system normally returns lipids absorbed from the small bowel via the thoracic duct, which ascends behind the esophagus to drain into the left brachiocephalic vein. If normal thoracic duct drainage is disrupted, either due to obstruction or rupture, chyle can leak and accumulate within the negative-pressured pleural space. In people on a normal diet, this fluid collection can sometimes be identified by its turbid, milky white appearance, since chyle contains emulsified triglycerides.
Thoracentesis, also known as thoracocentesis, pleural tap, needle thoracostomy, or needle decompression, is an invasive medical procedure to remove fluid or air from the pleural space for diagnostic or therapeutic purposes. A cannula, or hollow needle, is carefully introduced into the thorax, generally after administration of local anesthesia. The procedure was first performed by Morrill Wyman in 1850 and then described by Henry Ingersoll Bowditch in 1852.
Hemopneumothorax, or haemopneumothorax, is the condition of having both air (pneumothorax) and blood (hemothorax) in the chest cavity. A hemothorax, pneumothorax, or the combination of both can occur due to an injury to the lung or chest.

A surgical drain is a tube used to remove pus, blood or other fluids from a wound, body cavity, or organ. They are commonly placed by surgeons or interventional radiologists after procedures or some types of injuries, but they can also be used as an intervention for decompression. There are several types of drains, and selection of which to use often depends on the placement site and how long the drain is needed.
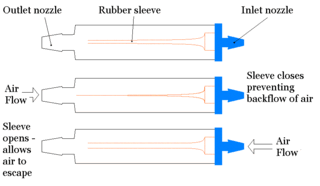
In respiratory medicine, a flutter valve is a one-way check valve used to prevent airflow back into a chest tube, and usually is applied to drain air from a pneumothorax. The design of the flutter valve features a rubber sleeve in a plastic case, where the rubber sleeve is arranged so that when air flows through the valve the sleeve opens and allows the outwards airflow from the body of the patient; however, when the airflow is reversed, the rubber sleeve closes and halts backwards airflow into the body of the patient.
A thoracostomy is a small incision of the chest wall, with maintenance of the opening for drainage. It is most commonly used for the treatment of a pneumothorax. This is performed by physicians, paramedics, and nurses usually via needle thoracostomy or an incision into the chest wall with the insertion of a thoracostomy tube or with a hemostat and the provider's finger.

A pulmonary laceration is a chest injury in which lung tissue is torn or cut. An injury that is potentially more serious than pulmonary contusion, pulmonary laceration involves disruption of the architecture of the lung, while pulmonary contusion does not. Pulmonary laceration is commonly caused by penetrating trauma but may also result from forces involved in blunt trauma such as shear stress. A cavity filled with blood, air, or both can form. The injury is diagnosed when collections of air or fluid are found on a CT scan of the chest. Surgery may be required to stitch the laceration, to drain blood, or even to remove injured parts of the lung. The injury commonly heals quickly with few problems if it is given proper treatment; however it may be associated with scarring of the lung or other complications.

Subcutaneous emphysema occurs when gas or air accumulates and seeps under the skin, where normally no gas should be present. Subcutaneous refers to the subcutaneous tissue, and emphysema refers to trapped air pockets. Since the air generally comes from the chest cavity, subcutaneous emphysema usually occurs around the upper torso, such as on the chest, neck, face, axillae and arms, where it is able to travel with little resistance along the loose connective tissue within the superficial fascia. Subcutaneous emphysema has a characteristic crackling-feel to the touch, a sensation that has been described as similar to touching warm Rice Krispies. This sensation of air under the skin is known as subcutaneous crepitation, a form of crepitus.

The Eloesser flap is a surgical procedure developed by Dr. Leo Eloesser in 1935 at the San Francisco General Hospital. It was originally intended to aid with drainage of tuberculous empyemas, since at the time there were no effective medications to treat tuberculosis. The procedure was used extensively until the development of effective chemotherapy for tuberculosis in the late 1940s and early 1950s. It is still used occasionally for chronic empyemas.
Chest drains are surgical drains placed within the pleural space to facilitate removal of unwanted substances in order to preserve respiratory functions and hemodynamic stability. Some chest drains may utilize a flutter valve to prevent retrograde flow, but those that do not have physical valves employ a water trap seal design, often aided by continuous suction from a wall suction or a portable vacuum pump.

Mediastinal shift is an abnormal movement of the mediastinal structures toward one side of the chest cavity. A shift indicates a severe imbalance of pressures inside the chest. Mediastinal shifts are generally caused by increased lung volume, decreased lung volume, or abnormalities in the pleural space. Additionally, masses inside the mediastinum or musculoskeletal abnormalities can also lead to abnormal mediastinal arrangement. Typically, these shifts are observed on x-ray but also on computed tomography (CT) or magnetic resonance imaging (MRI). On chest x-ray, tracheal deviation, or movement of the trachea away from its midline position can be used as a sign of a shift. Other structures like the heart can also be used as reference points. Below are examples of pathologies that can cause a mediastinal shift and their appearance.

Lung surgery is a type of thoracic surgery involving the repair or removal of lung tissue, and can be used to treat a variety of conditions ranging from lung cancer to pulmonary hypertension. Common operations include anatomic and nonanatomic resections, pleurodesis and lung transplants. Though records of lung surgery date back to the Classical Age, new techniques such as VATS continue to be developed.






















