Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.
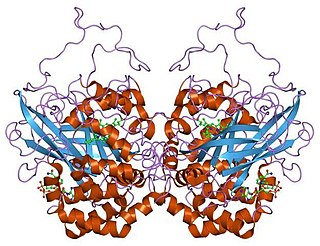
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cell from oxidative damage by reactive oxygen species (ROS). Catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of hydrogen peroxide molecules to water and oxygen each second.

Peroxidases or peroxide reductases are a large group of enzymes which play a role in various biological processes. They are named after the fact that they commonly break up peroxides.

Heme, or haem, is a ring-shaped iron-containing molecular component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver.

Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors.

Cytochrome c peroxidase, or CCP, is a water-soluble heme-containing enzyme of the peroxidase family that takes reducing equivalents from cytochrome c and reduces hydrogen peroxide to water:
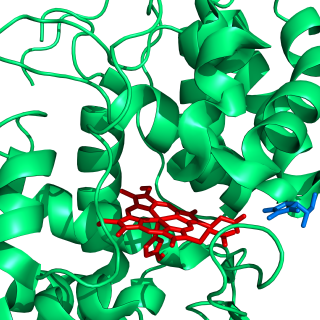
Ascorbate peroxidase (or L-ascorbate peroxidase, APX or APEX) (EC 1.11.1.11) is an enzyme that catalyzes the chemical reaction
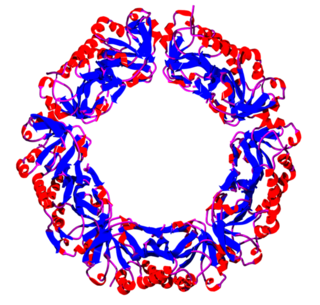
Peroxiredoxins are a ubiquitous family of antioxidant enzymes that also control cytokine-induced peroxide levels and thereby mediate signal transduction in mammalian cells. The family members in humans are PRDX1, PRDX2, PRDX3, PRDX4, PRDX5, and PRDX6. The physiological importance of peroxiredoxins is indicated by their relative abundance. Their function is the reduction of peroxides, specifically hydrogen peroxide, alkyl hydroperoxides, and peroxynitrite.
Chloride peroxidase (EC 1.11.1.10) is a family of enzymes that catalyzes the chlorination of organic compounds. This enzyme combines the inorganic substrates chloride and hydrogen peroxide to produce the equivalent of Cl+, which replaces a proton in hydrocarbon substrate:
In enzymology, a lignin peroxidase (EC 1.11.1.14) is an enzyme that catalyzes the chemical reaction
In enzymology, a manganese peroxidase (EC 1.11.1.13) is an enzyme that catalyzes the chemical reaction
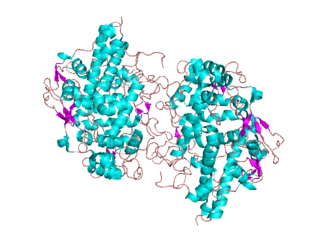
Animal heme-dependent peroxidases is a family of peroxidases. Peroxidases are found in bacteria, fungi, plants and animals. On the basis of sequence similarity, a number of animal heme peroxidases can be categorized as members of a superfamily: myeloperoxidase (MPO); eosinophil peroxidase (EPO); lactoperoxidase (LPO); thyroid peroxidase (TPO); prostaglandin H synthase (PGHS); and peroxidasin.
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment.
The ascorbate-glutathione cycle, sometimes Foyer-Halliwell-Asada pathway, is a metabolic pathway that detoxifies hydrogen peroxide (H2O2), a reactive oxygen species that is produced as a waste product in metabolism. The cycle involves the antioxidant metabolites: ascorbate, glutathione and NADPH and the enzymes linking these metabolites.

In molecular biology, the DyP-type peroxidase family is a family of haem peroxidase enzymes. Haem peroxidases were originally divided into two superfamilies, namely, the animal peroxidases and the plant peroxidases, which include fungal and bacterial peroxidases. The DyP family constitutes a novel class of haem peroxidase. Because these enzymes were derived from fungal sources, the DyP family was thought to be structurally related to the class II secretory fungal peroxidases. However, the DyP family exhibits only low sequence similarity to classical fungal peroxidases, such as LiP and MnP, and does not contain the conserved proximal and distal histidines and an essential arginine found in other plant peroxidase superfamily members.
Versatile peroxidase (EC 1.11.1.16, VP, hybrid peroxidase, polyvalent peroxidase) is an enzyme with systematic name reactive-black-5:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
Dye-decolorizing peroxidase (EC 1.11.1.19, DyP, DyP-type peroxidase) is an enzyme with systematic name Reactive-Blue-5:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
Catalase-peroxidase (EC 1.11.1.21, katG (gene)) is an enzyme with systematic name donor:hydrogen-peroxide oxidoreductase. This enzyme catalyses the following chemical reaction
- donor + H2O2 ⇌ oxidized donor + 2 H2O
- 2 H2O2 ⇌ O2 + 2 H2O
Oxidation response is stimulated by a disturbance in the balance between the production of reactive oxygen species and antioxidant responses, known as oxidative stress. Active species of oxygen naturally occur in aerobic cells and have both intracellular and extracellular sources. These species, if not controlled, damage all components of the cell, including proteins, lipids and DNA. Hence cells need to maintain a strong defense against the damage. The following table gives an idea of the antioxidant defense system in bacterial system.

Eosinophil peroxidase is an enzyme found within the eosinophil granulocytes, innate immune cells of humans and mammals. This oxidoreductase protein is encoded by the gene EPX, expressed within these myeloid cells. EPO shares many similarities with its orthologous peroxidases, myeloperoxidase (MPO), lactoperoxidase (LPO), and thyroid peroxidase (TPO). The protein is concentrated in secretory granules within eosinophils. Eosinophil peroxidase is a heme peroxidase, its activities including the oxidation of halide ions to bacteriocidal reactive oxygen species, the cationic disruption of bacterial cell walls, and the post-translational modification of protein amino acid residues.










