Related Research Articles

Eosinophilia is a condition in which the eosinophil count in the peripheral blood exceeds 5×108/L (500/μL). Hypereosinophilia is an elevation in an individual's circulating blood eosinophil count above 1.5 × 109/L (i.e. 1,500/μL). The hypereosinophilic syndrome is a sustained elevation in this count above 1.5 × 109/L (i.e. 1,500/μL) that is also associated with evidence of eosinophil-based tissue injury.
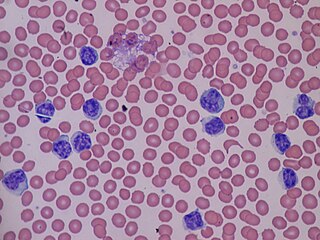
Lymphocytosis is an increase in the number or proportion of lymphocytes in the blood. Absolute lymphocytosis is the condition where there is an increase in the lymphocyte count beyond the normal range while relative lymphocytosis refers to the condition where the proportion of lymphocytes relative to white blood cell count is above the normal range. In adults, absolute lymphocytosis is present when the lymphocyte count is greater than 5000 per microliter (5.0 x 109/L), in older children greater than 7000 per microliter and in infants greater than 9000 per microliter. Lymphocytes normally represent 20% to 40% of circulating white blood cells. When the percentage of lymphocytes exceeds 40%, it is recognized as relative lymphocytosis.

Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes. Early on, there are typically no symptoms. Later, non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years.

Tumors of the hematopoietic and lymphoid tissues or tumours of the haematopoietic and lymphoid tissues are tumors that affect the blood, bone marrow, lymph, and lymphatic system. Because these tissues are all intimately connected through both the circulatory system and the immune system, a disease affecting one will often affect the others as well, making aplasia, myeloproliferation and lymphoproliferation closely related and often overlapping problems. While uncommon in solid tumors, chromosomal translocations are a common cause of these diseases. This commonly leads to a different approach in diagnosis and treatment of hematological malignancies. Hematological malignancies are malignant neoplasms ("cancer"), and they are generally treated by specialists in hematology and/or oncology. In some centers "hematology/oncology" is a single subspecialty of internal medicine while in others they are considered separate divisions. Not all hematological disorders are malignant ("cancerous"); these other blood conditions may also be managed by a hematologist.

Monoclonal gammopathy of undetermined significance (MGUS) is a plasma cell dyscrasia in which plasma cells or other types of antibody-producing cells secrete a myeloma protein, i.e. an abnormal antibody, into the blood; this abnormal protein is usually found during standard laboratory blood or urine tests. MGUS resembles multiple myeloma and similar diseases, but the levels of antibodies are lower, the number of plasma cells in the bone marrow is lower, and it rarely has symptoms or major problems. However, since MGUS can lead to multiple myeloma, which develops at the rate of about 1.5% a year, or other symptomatic conditions, yearly monitoring is recommended.
Lymphoproliferative disorders (LPDs) refer to a specific class of diagnoses, comprising a group of several conditions, in which lymphocytes are produced in excessive quantities. These disorders primarily present in patients who have a compromised immune system. Due to this factor, there are instances of these conditions being equated with "immunoproliferative disorders"; although, in terms of nomenclature, lymphoproliferative disorders are a subclass of immunoproliferative disorders—along with hypergammaglobulinemia and paraproteinemias.
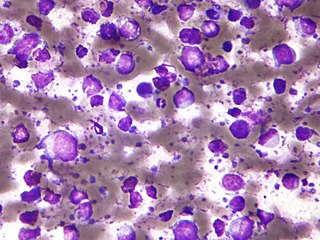
The B-cell lymphomas are types of lymphoma affecting B cells. Lymphomas are "blood cancers" in the lymph nodes. They develop more frequently in older adults and in immunocompromised individuals.
Splenic marginal zone lymphoma (SMZL) is a type of cancer made up of B-cells that replace the normal architecture of the white pulp of the spleen. The neoplastic cells are both small lymphocytes and larger, transformed lymphoblasts, and they invade the mantle zone of splenic follicles and erode the marginal zone, ultimately invading the red pulp of the spleen. Frequently, the bone marrow and splenic hilar lymph nodes are involved along with the peripheral blood. The neoplastic cells circulating in the peripheral blood are termed villous lymphocytes due to their characteristic appearance.
Richter's transformation (RT), also known as Richter's syndrome, is the conversion of chronic lymphocytic leukemia (CLL) or its variant, small lymphocytic lymphoma (SLL), into a new and more aggressively malignant disease. CLL is the circulation of malignant B lymphocytes with or without the infiltration of these cells into lymphatic or other tissues while SLL is the infiltration of these malignant B lymphocytes into lymphatic and/or other tissues with little or no circulation of these cells in the blood. CLL along with its SLL variant are grouped together in the term CLL/SLL.

B-cell prolymphocytic leukemia, referred to as B-PLL, is a rare blood cancer. It is a more aggressive, but still treatable, form of leukemia.
Plasma cell dyscrasias are a spectrum of progressively more severe monoclonal gammopathies in which a clone or multiple clones of pre-malignant or malignant plasma cells over-produce and secrete into the blood stream a myeloma protein, i.e. an abnormal monoclonal antibody or portion thereof. The exception to this rule is the disorder termed non-secretory multiple myeloma; this disorder is a form of plasma cell dyscrasia in which no myeloma protein is detected in serum or urine of individuals who have clear evidence of an increase in clonal bone marrow plasma cells and/or evidence of clonal plasma cell-mediated tissue injury. Here, a clone of plasma cells refers to group of plasma cells that are abnormal in that they have an identical genetic identity and therefore are descendants of a single genetically distinct ancestor cell.
Hematologic diseases are disorders which primarily affect the blood & blood-forming organs. Hematologic diseases include rare genetic disorders, anemia, HIV, sickle cell disease & complications from chemotherapy or transfusions.

Marginal zone B-cell lymphomas, also known as marginal zone lymphomas (MZLs), are a heterogeneous group of lymphomas that derive from the malignant transformation of marginal zone B-cells. Marginal zone B cells are innate lymphoid cells that normally function by rapidly mounting IgM antibody immune responses to antigens such as those presented by infectious agents and damaged tissues. They are lymphocytes of the B-cell line that originate and mature in secondary lymphoid follicles and then move to the marginal zones of mucosa-associated lymphoid tissue, the spleen, or lymph nodes. Mucosa-associated lymphoid tissue is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body such as the gastrointestinal tract, mouth, nasal cavity, pharynx, thyroid gland, breast, lung, salivary glands, eye, skin and the human spleen.
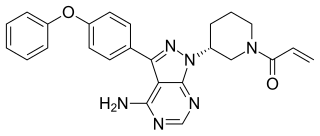
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that inhibits B-cell proliferation and survival by irreversibly binding the protein Bruton's tyrosine kinase (BTK). Blocking BTK inhibits the B-cell receptor pathway, which is often aberrantly active in B cell cancers. Ibrutinib is therefore used to treat such cancers, including mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia. Ibrutinib also binds to C-terminal Src Kinases. These are off-target receptors for the BTK inhibitor. Ibrutinib binds to these receptors and inhibits the kinase from promoting cell differentiation and growth. This leads to many different side effects like left atrial enlargement and atrial fibrillation during the treatment of Chronic Lymphocytic Leukemia.
BENTA disease is a rare genetic disorder of the immune system. BENTA stands for "B cell expansion with NF-κB and T cell anergy" and is caused by germline heterozygous gain-of-function mutations in the gene CARD11. This disorder is characterized by polyclonal B cell lymphocytosis with onset in infancy, splenomegaly, lymphadenopathy, mild immunodeficiency, and increased risk of lymphoma. Investigators Andrew L. Snow and Michael J. Lenardo at the National Institute of Allergy and Infectious Diseases at the U.S. National Institutes of Health first characterized BENTA disease in 2012. Dr. Snow's current laboratory at the Uniformed Services University of the Health Sciences is now actively studying this disorder.

Venetoclax, sold under the brand names Venclexta and Venclyxto, is a medication used to treat adults with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), or acute myeloid leukemia (AML).
Lymphocyte-variant hypereosinophilia is a rare disorder in which eosinophilia or hypereosinophilia is caused by an aberrant population of lymphocytes. These aberrant lymphocytes function abnormally by stimulating the proliferation and maturation of bone marrow eosinophil-precursor cells termed colony forming unit-Eosinophils or CFU-Eos.
Epstein–Barr virus–associated lymphoproliferative diseases are a group of disorders in which one or more types of lymphoid cells, i.e. B cells, T cells, NK cells, and histiocytic-dendritic cells, are infected with the Epstein–Barr virus (EBV). This causes the infected cells to divide excessively, and is associated with the development of various non-cancerous, pre-cancerous, and cancerous lymphoproliferative disorders (LPDs). These LPDs include the well-known disorder occurring during the initial infection with the EBV, infectious mononucleosis, and the large number of subsequent disorders that may occur thereafter. The virus is usually involved in the development and/or progression of these LPDs although in some cases it may be an "innocent" bystander, i.e. present in, but not contributing to, the disease.
In situ lymphoid neoplasia is a precancerous condition newly classified by the World Health Organization in 2016. The Organization recognized two subtypes of ISLN: in situ follicular neoplasia (ISFN) and in situ mantle cell neoplasia (ISMCL). ISFN and ISMCL are pathological accumulations of lymphocytes in the germinal centers and mantle zones, respectively, of the follicles that populate lymphoid organs such as lymph nodes. These lymphocytes are monoclonal B-cells that may develop into follicular (FL) and mantle cell (MCL) lymphomas, respectively.

Indolent lymphoma, also known as low-grade lymphoma, is a group of slow-growing non-Hodgkin lymphomas (NHLs). Because they spread slowly, they tend to have fewer signs and symptoms when first diagnosed and may not require immediate treatment. Symptoms can include swollen but painless lymph nodes, unexplained fever, and unintended weight loss.
References
- ↑ Jaffe ES (January 2019). "Diagnosis and classification of lymphoma: Impact of technical advances". Seminars in Hematology. 56 (1): 30–36. doi: 10.1053/j.seminhematol.2018.05.007 . PMC 7394061 . PMID 30573042.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Angelillo P, Capasso A, Ghia P, Scarfò L (December 2018). "Monoclonal B-cell lymphocytosis: Does the elderly patient need a specialistic approach?". European Journal of Internal Medicine. 58: 2–6. doi:10.1016/j.ejim.2018.09.006. PMID 30268574. S2CID 52892403.
- 1 2 3 4 5 6 7 8 9 10 11 12 Choi SM, O'Malley DP (December 2018). "Diagnostically relevant updates to the 2017 WHO classification of lymphoid neoplasms". Annals of Diagnostic Pathology. 37: 67–74. doi:10.1016/j.anndiagpath.2018.09.011. PMID 30308438. S2CID 52963674.
- 1 2 Hallek M (September 2017). "Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment". American Journal of Hematology. 92 (9): 946–965. doi: 10.1002/ajh.24826 . PMID 28782884.
- ↑ Tresckow JV, Eichhorst B, Bahlo J, Hallek M (January 2019). "The Treatment of Chronic Lymphatic Leukemia". Deutsches Ärzteblatt International. 116 (4): 41–46. doi:10.3238/arztebl.2019.0041. PMC 6415618 . PMID 30855005.
- 1 2 3 4 5 6 7 8 9 10 11 Xochelli A, Oscier D, Stamatopoulos K (2017). "Clonal B-cell lymphocytosis of marginal zone origin". Best Practice & Research. Clinical Haematology. 30 (1–2): 77–83. doi:10.1016/j.beha.2016.08.028. PMID 28288720.
- ↑ Hallek M, Cheson BD, Catovsky D, et al. (June 2008). "Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines". Blood. 111 (12): 5446–56. doi:10.1182/blood-2007-06-093906. PMC 2972576 . PMID 18216293.
- ↑ Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES (May 2016). "The 2016 revision of the World Health Organization classification of lymphoid neoplasms". Blood. 127 (20): 2375–90. doi:10.1182/blood-2016-01-643569. PMC 4874220 . PMID 26980727.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Strati P, Shanafelt TD (July 2015). "Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification". Blood. 126 (4): 454–62. doi:10.1182/blood-2015-02-585059. PMC 4624440 . PMID 26065657.
- ↑ Marti G, Abbasi F, Raveche E, Rawstron AC, Ghia P, Aurran T, Caporaso N, Shim YK, Vogt RF (December 2007). "Overview of monoclonal B-cell lymphocytosis". Br. J. Haematol. 139 (5): 701–8. doi:10.1111/j.1365-2141.2007.06865.x. PMID 18021084.
- 1 2 Wiernik PH (February 2015). "Familial leukemias". Current Treatment Options in Oncology. 16 (2): 8. doi:10.1007/s11864-014-0323-3. PMID 25762123. S2CID 9926252.
- ↑ Milpied P, Nadel B, Roulland S (July 2015). "Premalignant cell dynamics in indolent B-cell malignancies". Current Opinion in Hematology. 22 (4): 388–96. doi:10.1097/MOH.0000000000000159. PMID 26049761. S2CID 26080460.
- ↑ Rodríguez D, Bretones G, Arango JR, Valdespino V, Campo E, Quesada V, López-Otín C (March 2015). "Molecular pathogenesis of CLL/SLL and its evolution". International Journal of Hematology. 101 (3): 219–28. doi: 10.1007/s12185-015-1733-0 . PMID 25630433.
- ↑ Popp HD, Flach J, Brendel S, Ruppenthal S, Kleiner H, Seifarth W, Schneider S, Schulze TJ, Weiss C, Wenz F, Hofmann WK, Fabarius A (March 2019). "Accumulation of DNA damage and alteration of the DNA damage response in monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia". Leukemia & Lymphoma. 60 (3): 795–804. doi:10.1080/10428194.2018.1498494. PMID 30376743. S2CID 53110926.
- ↑ Rachel JM, Zucker ML, Fox CM, Plapp FV, Menitove JE, Abbasi F, Marti GE (December 2007). "Monoclonal B-cell lymphocytosis in blood donors". British Journal of Haematology. 139 (5): 832–6. doi:10.1111/j.1365-2141.2007.06870.x. PMID 17961190. S2CID 32218085.
- ↑ Stetler-Stevenson M (February 2014). "Monoclonal B-cell lymphocytosis in donors". Blood. 123 (9): 1281–2. doi:10.1182/blood-2014-01-546739. PMC 3938144 . PMID 24578489.
- ↑ Hjalgrim H, Rostgaard K, Vasan SK, Ullum H, Erikstrup C, Pedersen OB, Nielsen KR, Titlestad KE, Melbye M, Nyrén O, Edgren G (October 2015). "No evidence of transmission of chronic lymphocytic leukemia through blood transfusion". Blood. 126 (17): 2059–61. doi: 10.1182/blood-2015-03-632844 . PMID 26302757.
- 1 2 Raess PW, Mintzer D, Husson M, Nakashima MO, Morrissette JJ, Daber R, Bagg A (October 2013). "BRAF V600E is also seen in unclassifiable splenic B-cell lymphoma/leukemia, a potential mimic of hairy cell leukemia". Blood. 122 (17): 3084–5. doi: 10.1182/blood-2013-07-513523 . PMID 24159168.
- ↑ Wotherspoon A, Attygalle A, Mendes LS (December 2015). "Bone marrow and splenic histology in hairy cell leukaemia". Best Practice & Research. Clinical Haematology. 28 (4): 200–7. doi:10.1016/j.beha.2015.10.019. PMID 26614898.
- ↑ Oishi N, Montes-Moreno S, Feldman AL (January 2018). "In situ neoplasia in lymph node pathology". Seminars in Diagnostic Pathology. 35 (1): 76–83. doi:10.1053/j.semdp.2017.11.001. PMID 29129357.
- ↑ Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, Schleinitz TA, Caporaso N (August 2005). "Diagnostic criteria for monoclonal B-cell lymphocytosis". British Journal of Haematology. 130 (3): 325–32. doi: 10.1111/j.1365-2141.2005.05550.x . PMID 16042682.