
Coeliac disease or celiac disease is a long-term autoimmune disorder, primarily affecting the small intestine, where individuals develop intolerance to gluten, present in foods such as wheat, rye and barley. Classic symptoms include gastrointestinal problems such as chronic diarrhoea, abdominal distention, malabsorption, loss of appetite, and among children failure to grow normally. This often begins between six months and two years of age. Non-classic symptoms are more common, especially in people older than two years. There may be mild or absent gastrointestinal symptoms, a wide number of symptoms involving any part of the body, or no obvious symptoms. Coeliac disease was first described in childhood; however, it may develop at any age. It is associated with other autoimmune diseases, such as Type 1 diabetes mellitus and Hashimoto's thyroiditis, among others.

The human leukocyte antigen (HLA) system or complex is a complex of genes on chromosome 6 in humans which encode cell-surface proteins responsible for regulation of the immune system. The HLA system is also known as the human version of the major histocompatibility complex (MHC) found in many animals.

Anaplastic large-cell lymphoma (ALCL) refers to a group of non-Hodgkin lymphomas in which aberrant T cells proliferate uncontrollably. Considered as a single entity, ALCL is the most common type of peripheral lymphoma and represents ~10% of all peripheral lymphomas in children. The incidence of ALCL is estimated to be 0.25 cases per 100,000 people in the United States of America. There are four distinct types of anaplastic large-cell lymphomas that on microscopic examination share certain key histopathological features and tumor marker proteins. However, the four types have very different clinical presentations, gene abnormalities, prognoses, and/or treatments.

Intravascular lymphomas (IVL) are rare cancers in which malignant lymphocytes proliferate and accumulate within blood vessels. Almost all other types of lymphoma involve the proliferation and accumulation of malignant lymphocytes in lymph nodes, other parts of the lymphatic system, and various non-lymphatic organs but not in blood vessels.

HLA-DQ (DQ) is a cell surface receptor protein found on antigen-presenting cells. It is an αβ heterodimer of type MHC class II. The α and β chains are encoded by two loci, HLA-DQA1 and HLA-DQB1, that are adjacent to each other on chromosome band 6p21.3. Both α-chain and β-chain vary greatly. A person often produces two α-chain and two β-chain variants and thus 4 isoforms of DQ. The DQ loci are in close genetic linkage to HLA-DR, and less closely linked to HLA-DP, HLA-A, HLA-B and HLA-C.
HLA DR3-DQ2 is double serotype that specifically recognizes cells from individuals who carry a multigene HLA DR, DQ haplotype. Certain HLA DR and DQ genes have known involvement in autoimmune diseases. DR3-DQ2, a multigene haplotype, stands out in prominence because it is a factor in several prominent diseases, namely coeliac disease and juvenile diabetes. In coeliac disease, the DR3-DQ2 haplotype is associated with highest risk for disease in first degree relatives, highest risk is conferred by DQA1*0501:DQB1*0201 homozygotes and semihomozygotes of DQ2, and represents the overwhelming majority of risk. HLA DR3-DQ2 encodes DQ2.5cis isoform of HLA-DQ, this isoform is described frequently as 'the DQ2 isoform', but in actuality there are two major DQ2 isoform. The DQ2.5 isoform, however, is many times more frequently associated with autoimmune disease, and as a result to contribution of DQ2.2 is often ignored.
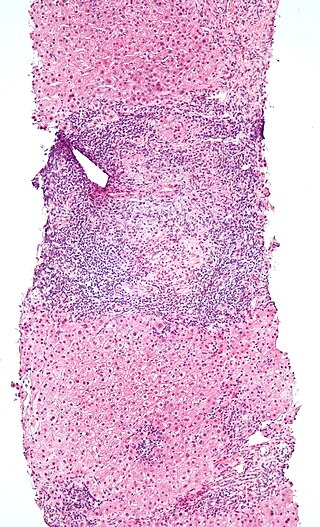
Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract. However, unlike other T cells, IELs do not need priming. Upon encountering antigens, they immediately release cytokines and cause killing of infected target cells. In the GI tract, they are components of gut-associated lymphoid tissue (GALT).

Gluten-related disorders is the term for the diseases triggered by gluten, including celiac disease (CD), non-celiac gluten sensitivity (NCGS), gluten ataxia, dermatitis herpetiformis (DH) and wheat allergy. The umbrella category has also been referred to as gluten intolerance, though a multi-disciplinary physician-led study, based in part on the 2011 International Coeliac Disease Symposium, concluded that the use of this term should be avoided due to a lack of specificity.
Gluten-sensitive enteropathy–associated conditions are comorbidities or complications of gluten-related gastrointestinal distress. GSE has key symptoms typically restricted to the bowel and associated tissues; however, there are a wide variety of associated conditions. These include bowel disorders, eosinophilic gastroenteritis and increase with coeliac disease (CD) severity. With some early onset and a large percentage of late onset disease, other disorders appear prior to the coeliac diagnosis or allergic-like responses markedly increased in GSE. Many of these disorders persist on a strict gluten-free diet, and are thus independent of coeliac disease after triggering. For example, autoimmune thyroiditis is a common finding with GSE.
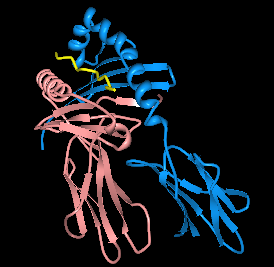
HLA-DQ2 (DQ2) is a serotype group within HLA-DQ (DQ) serotyping system. The serotype is determined by the antibody recognition of β2 subset of DQ β-chains. The β-chain of DQ is encoded by HLA-DQB1 locus and DQ2 are encoded by the HLA-DQB1*02 allele group. This group currently contains two common alleles, DQB1*0201 and DQB1*0202. HLA-DQ2 and HLA-DQB1*02 are almost synonymous in meaning. DQ2 β-chains combine with α-chains, encoded by genetically linked HLA-DQA1 alleles, to form the cis-haplotype isoforms. These isoforms, nicknamed DQ2.2 and DQ2.5, are also encoded by the DQA1*0201 and DQA1*0501 genes, respectively.

HLA-DQ7 (DQ7) is an HLA-DQ serotype that recognizes the common HLA DQB1*0301 and the less common HLA DQB1*0304 gene products. DQ7 is a form of 'split antigen' of the broad antigen group DQ3 which also contains DQ8 and DQ9.
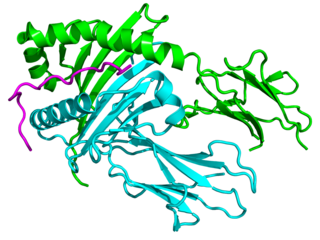
Major histocompatibility complex, class II, DQ beta 1, also known as HLA-DQB1, is a human gene and also denotes the genetic locus that contains this gene. The protein encoded by this gene is one of two proteins that are required to form the DQ heterodimer, a cell surface receptor essential to the function of the immune system.
Oat sensitivity represents a sensitivity to the proteins found in oats, Avena sativa. Sensitivity to oats can manifest as a result of allergy to oat seed storage proteins either inhaled or ingested. A more complex condition affects individuals who have gluten-sensitive enteropathy in which there is an autoimmune response to avenin, the glutinous protein in oats similar to the gluten within wheat. Sensitivity to oat foods can also result from their frequent contamination by wheat, barley, or rye particles.
The immunochemistry of Triticeae glutens is important in several inflammatory diseases. It can be subdivided into innate responses, class II mediated presentation, class I mediated stimulation of killer cells, and antibody recognition. The responses to gluten proteins and polypeptide regions differs according to the type of gluten sensitivity. The response is also dependent on the genetic makeup of the human leukocyte antigen genes. In gluten sensitive enteropathy, there are four types of recognition, innate immunity, HLA-DQ, and antibody recognition of gliadin and transglutaminase. With idiopathic gluten sensitivity only antibody recognition to gliadin has been resolved. In wheat allergy, the response pathways are mediated through IgE against other wheat proteins and other forms of gliadin.
HLA A1-B8-DR3-DQ2 haplotype is a multigene haplotype that covers a majority of the human major histocompatibility complex on chromosome 6. A multigene haplotype is set of inherited alleles covering several genes, or gene-alleles; common multigene haplotypes are generally the result of descent by common ancestry. Chromosomal recombination fragments multigene haplotypes as the distance to that ancestor increases in number of generations.

Extranodal NK/T-cell lymphoma, nasal type (ENKTCL-NT) is a rare type of lymphoma that commonly involves midline areas of the nasal cavity, oral cavity, and/or pharynx At these sites, the disease often takes the form of massive, necrotic, and extremely disfiguring lesions. However, ENKTCL-NT can also involve the eye, larynx, lung, gastrointestinal tract, skin, and various other tissues. ENKTCL-NT mainly affects adults; it is relatively common in Asia and to lesser extents Mexico, Central America, and South America but is rare in Europe and North America. In Korea, ENKTCL-NT often involves the skin and is reported to be the most common form of cutaneous lymphoma after mycosis fungoides.
Duodenal lymphocytosis, sometimes called lymphocytic duodenitis, lymphocytic duodenosis, or duodenal intraepithelial lymphocytosis, is a condition where an increased number of intra-epithelial lymphocytes is seen in biopsies of the duodenal mucosa when these are examined microscopically. This form of lymphocytosis is often a feature of coeliac disease but may be found in other disorders.
Natural killer cell enteropathy, also termed NK cell enteropathy (NKCE), and a closely related disorder, lymphomatoid gastropathy (LG), are non-malignant diseases in which one type of lymphocyte, the natural killer cell, proliferates excessively in the gastrointestinal tract. This proliferation causes red, sore-like spots, raised lesions, erosions, and ulcers in the mucosal layer surrounding the GI tract lumen. Both disorders cause either no or only vague symptoms of GI tract disturbances such as nausea, vomiting, and bleeding.
Indolent T cell lymphoproliferative disorder of the gastrointestinal tract or Indolent T cell lymphoproliferative disorder of the GI tract (ITCLD-GT) is a rare and recently recognized disorder in which mature T cell lymphocytes accumulation abnormally in the gastrointestinal tract. This accumulation causes various lesions in the mucosal layer lining the GI tract. Individuals with ITCLD-GT commonly complain of chronic GI tract symptoms such as nausea, vomiting, diarrhea, abdominal pain, and rectal bleeding.
Monomorphic epitheliotropic intestinal T cell lymphoma (MEITL) is an extremely rare peripheral T-cell lymphoma that involves the malignant proliferation of a type of lymphocyte, the T cell, in the gastrointestinal tract. Over time, these T cells commonly spread throughout the mucosal lining of a portion of the GI tract, lead to GI tract nodules and ulcerations, and cause symptoms such as abdominal pain, weight loss, diarrhea, obstruction, bleeding, and/or perforation.








