
Selenium is a chemical element; it has symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.
A selenide is a chemical compound containing a selenium with oxidation number of −2. Similar to sulfide, selenides occur both as inorganic compounds and as organic derivatives, which are called organoselenium compound.

Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or 'leaking gas', but smells of rotten eggs at higher concentrations.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.

Selenous acid is the chemical compound with the formula H2SeO3. Structurally, it is more accurately described by O=Se(OH)2. It is the principal oxoacid of selenium; the other being selenic acid.

Sodium sulfide is a chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene. It is named for Paul Grieco.
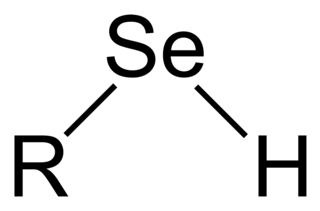
Selenols are organic compounds that contain the functional group with the connectivity C–Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. A well-known selenol is the amino acid selenocysteine.

Hydrogen telluride is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.

Sodium aluminium hydride or sodium alumanuide is an inorganic compound with the chemical formula NaAlH4. It is a white pyrophoric solid that dissolves in tetrahydrofuran (THF), but not in diethyl ether or hydrocarbons. It has been evaluated as an agent for the reversible storage of hydrogen and it is used as a reagent for the chemical synthesis of organic compounds. Similar to lithium aluminium hydride, it is a salt consisting of separated sodium cations and tetrahedral AlH−
4 anions.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.

Sodium selenide is an inorganic compound of sodium and selenium with the chemical formula Na2Se.
Selenium monochloride or diselenium dichloride is an inorganic compound with the formula Se2Cl2. Although a common name for the compound is selenium monochloride, reflecting its empirical formula, IUPAC does not recommend that name, instead preferring the more descriptive diselenium dichloride.

Selenopyrylium is an aromatic heterocyclic compound consisting of a six-membered ring with five carbon atoms and a positively charged selenium atom.

Carbonyl selenide is a chemical compound with the chemical formula O=C=Se. It is a linear molecule that is primarily of interest for research purposes.

In chemistry, a polyselenide usually refers to anions of the formula (Sen)2-, where Se is the atomic symbol for the element selenium. Many main group and transition metals form complexes with polyselenide anions.















