
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.
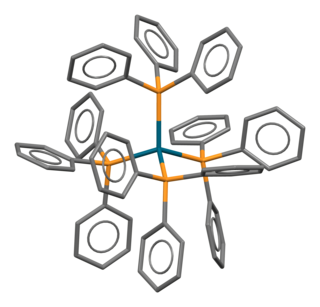
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound [Pd(P(C6H5)3)4], often abbreviated Pd(PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a common symmetrical bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). It is one of the more common phosphine oxides. This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.
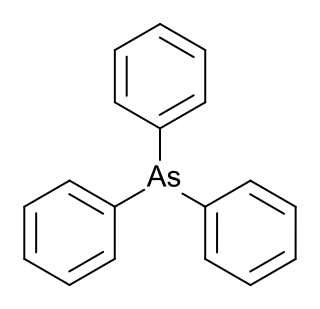
Triphenylarsine is the chemical compound with the formula As(C6H5)3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in homogeneous catalysts.

Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts.

Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into molecules, which includes many ligands. Like other halophosphines, Ph2PCl is reactive with many nucleophiles such as water and easily oxidized even by air.
Phenylphosphine is an organophosphorus compound with the chemical formula C6H5PH2. It is the phosphorus analog of aniline. Like other primary phosphines, phenylphosphine has an intense penetrating odor and is highly oxidizable. It is mainly used as a precursor to other organophosphorus compounds. It can function as a ligand in coordination chemistry.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

Dichlorobis(triphenylphosphine)nickel(II) refers to a pair of metal phosphine complexes with the formula NiCl2[P(C6H5)3]2. The compound exists as two isomers, a paramagnetic dark blue solid and a diamagnetic red solid. These complexes function as catalysts for organic synthesis.
Hydrophosphination is the insertion of a carbon-carbon multiple bond into a phosphorus-hydrogen bond forming a new phosphorus-carbon bond. Like other hydrofunctionalizations, the rate and regiochemistry of the insertion reaction is influenced by the catalyst. Catalysts take many forms, but most prevalent are bases and free-radical initiators. Most hydrophosphinations involve reactions of phosphine (PH3).
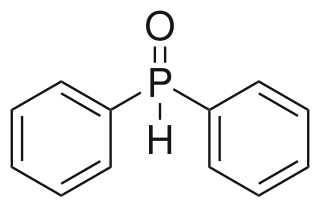
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents.

Lithium diphenylphosphide contains lithium and the organophosphorus anion with the formula (C6H5)2PLi. It is an air-sensitive solid that is used in the preparation of diphenylphosphino compounds. As an ether complex, the lithium salt is dark red.

Bis(triphenylphosphine)rhodium carbonyl chloride is the organorhodium complex with the formula [RhCl(CO)(PPh3)2]. This complex of rhodium(I) is a bright yellow, air-stable solid. It is the Rh analogue of Vaska's complex, the corresponding iridium complex. With regards to its structure, the complex is square planar with mutually trans triphenylphosphine (PPh3) ligands. The complex is a versatile homogeneous catalyst.
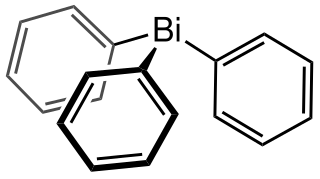
Triphenylbismuthine is an organobismuth compound with the formula Bi(C6H5)3. It is a white, air-stable solid that is soluble in organic solvents. It is prepared by treatment of bismuth trichloride with phenylmagnesium bromide.

















