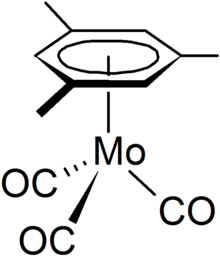Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.

An epoxide is a cyclic ether with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups. Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (η5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.
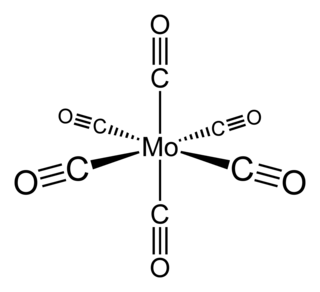
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.

Chromium carbonyl, also known as chromium hexacarbonyl, is the chemical compound with the formula Cr(CO)6. At room temperature the solid is stable to air, although it does have a high vapor pressure and sublimes readily. Cr(CO)6 is zerovalent, meaning that Cr has an oxidation state of zero, and it is a homoleptic complex, which means that all the ligands are identical. The complex is octahedral with Cr–C and C–O distances of 1.91 and 1.14 Å, respectively.

In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic covalent bonds to two arene ligands. The arenes have the formula CnHn, substituted derivatives (for example Cn(CH3)n) and heterocyclic derivatives (for example BCnHn+1). Because the metal is usually situated between the two rings, it is said to be "sandwiched". A special class of sandwich complexes are the metallocenes.

Tungsten hexacarbonyl (also called tungsten carbonyl) is the chemical compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.
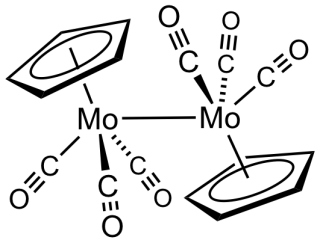
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5. A dark red solid, it has been the subject of much research although it has no practical uses.
Organochromium chemistry is a branch of organometallic chemistry that deals with organic compounds containing a chromium to carbon bond and their reactions. The field is of some relevance to organic synthesis. The relevant oxidation states for organochromium complexes encompass the entire range of possible oxidation states from –4 (d10) in Na4[Cr–IV(CO)4] to +6 (d0) in oxo-alkyl complexes like Cp*CrVI(=O)2Me.
In organometallic chemistry, a transition metal indenyl complex is a coordination compound that contains one or more indenyl ligands. The indenyl ligand is formally the anion derived from deprotonation of indene. The η5-indenyl ligand is related to the η5cyclopentadienyl anion (Cp), thus indenyl analogues of many cyclopentadienyl complexes are known. Indenyl ligands lack the 5-fold symmetry of Cp, so they exhibit more complicated geometries. Furthermore, some indenyl complexes also exist with only η3-bonding mode. The η5- and η3-bonding modes sometimes interconvert.

Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.
(Benzene)chromium tricarbonyl is an organometallic compound with the formula Cr(C6H6)(CO)3. This yellow crystalline solid compound is soluble in common nonpolar organic solvents. The molecule adopts a geometry known as “piano stool” because of the planar arrangement of the aryl group and the presence of three CO ligands as "legs" on the chromium-bond axis.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. While iron adopts oxidation states from Fe(−II) through to Fe(VII), Fe(IV) is the highest established oxidation state for organoiron species. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles. They are often called triazacyclohexanes or TACH's but this acronym is also applied to cis,cis-1,3,5-triaminocyclohexane
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.
Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes of are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene).