
Molybdenum is a chemical element; it has symbol Mo and atomic number 42. The name derived from Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.

Molybdenum disulfide is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is MoS
2.
In organic chemistry, a carbyne is a general term for any compound whose structure consists of an electrically neutral carbon atom connected by a single covalent bond and has three non-bonded electrons. The carbon atom has either one or three unpaired electrons, depending on its excitation state; making it a radical. The chemical formula can be written R−C· or R−C3·, or just CH.

In organic chemistry, olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.
Molybdenum trioxide describes a family of inorganic compounds with the formula MoO3(H2O)n where n = 0, 1, 2. The anhydrous compound is produced on the largest scale of any molybdenum compound since it is the main intermediate produced when molybdenum ores are purified. The anhydrous oxide is a precursor to molybdenum metal, an important alloying agent. It is also an important industrial catalyst. It is a yellow solid, although impure samples can appear blue or green.
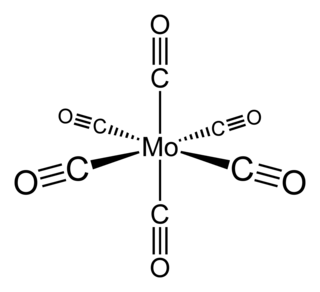
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium, tungsten, and seaborgium analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.
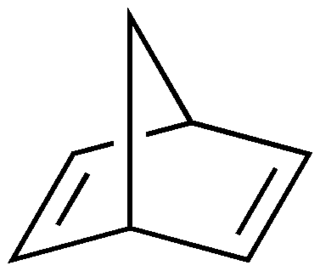
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize. Norbornadiene is also a useful dienophile in Diels-Alder reactions.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
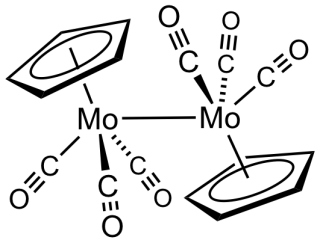
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5. A dark red solid, it has been the subject of much research although it has no practical uses.

Molybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. It is a colourless solid and melts just below room temperature and boils in 34 °C. It is one of the seventeen known binary hexafluorides.
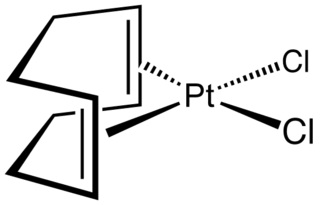
Dichloro(1,5-cyclooctadiene)platinum(II) (Pt(cod)Cl2) is an organometallic compound of platinum. This colourless solid is an entry point to other platinum compounds through the displacement of the cod and/or chloride ligands. It is one of several complexes of cycloocta-1,5-diene.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Molybdenum(II) acetate is a coordination compound with the formula Mo2(O2CCH3)4. It is a yellow, diamagnetic, air-stable solid that is slightly soluble in organic solvents. Molybdenum(II) acetate is an iconic example of a compound with a metal-metal quadruple bond.

Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.
In organometallic chemistry, a transition metal alkene complex is a coordination compound containing one or more alkene ligands. The inventory is large. Such compounds are intermediates in many catalytic reactions that convert alkenes to other organic products.
Ammonium dimolybdate (ADM) is the inorganic compound with the formula (NH4)2Mo2O7. It is a white, water-soluble solid. ADM is an intermediate in the production of molybdenum compounds from its ores. Roasting typical ore produces crude molybdenum(VI) oxides, which can be extracted into aqueous ammonia, affording ammonium molybdate. Heating solutions of ammonium molybdate gives ADM. Upon heating, solid ammonium dimolybdate decomposes to molybdenum trioxide:

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
In chemistry, metal vapor synthesis (MVS) is a method for preparing metal complexes by combining freshly produced metal atoms or small particles with ligands. In contrast to the high reactivity of such freshly produced metal atoms, bulk metals typically are unreactive toward neutral ligands. The method has been used to prepare compounds that cannot be prepared by traditional synthetic methods, e.g. Ti(η6-toluene)2. The technique relies on a reactor that evaporates the metal, allowing the vapor to impinge on a cold reactor wall that is coated with the organic ligand. The metal evaporates upon being heated resistively or irradiated with an electron beam. The apparatus operates under high vacuum. In a common implementation, the metal vapor and the organic ligand are co-condensed at liquid nitrogen temperatures.
















