Freon is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol propellants. These include chlorofluorocarbons and hydrofluorocarbons, both of which cause ozone depletion and contribute to global warming. 'Freon' is the brand name for the refrigerants R-12, R-13B1, R-22, R-410A, R-502, and R-503 manufactured by The Chemours Company, and so is not used to label all refrigerants of this type. They emit a strong smell similar to acetone.

Trichloroethylene (TCE) is a halocarbon with the formula C2HCl3, commonly used as an industrial degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste. Its IUPAC name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a volatile anesthetic and as an inhaled obstetrical analgesic in millions of patients. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.

A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.
Tetrafluoroethane is a fluoroalkane with two isomers:

Hydrofluorocarbons (HFCs) are man-made organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs), which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) which are presently being phased out. HFCs replaced older chlorofluorocarbons such as R-12 and hydrochlorofluorocarbons such as R-21. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection.

Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.
Difluoromethane, also called difluoromethylene, HFC-32Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH2F2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability. Due to the low melting and boiling point, (-136.0 °C and -51.6 °C respectively) contact with this compound may result in frostbite. In the United States, the Clean Air Act Section 111 on Volatile Organic Compounds (VOC) has listed difluoromethane as an exception (since 1997) from the definition of VOC due to its low production of tropospheric ozone. Difluoromethane is commonly used in endothermic processes such as refrigeration or air conditioning.
1,1,1,2-Tetrafluoroethane (also known as norflurane (INN), R-134a, Klea 134a,Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, HFA-134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties similar to R-12 (dichlorodifluoromethane) but with insignificant ozone depletion potential and a lower 100-year global warming potential (1,430, compared to R-12's GWP of 10,900). It has the formula CF3CH2F and a boiling point of −26.3 °C (−15.34 °F) at atmospheric pressure. R-134a cylinders are colored light blue. A phaseout and transition to HFO-1234yf and other refrigerants, with GWPs similar to CO2, began in 2012 within the automotive market.

A gas duster, also known as tinned wind or compressed air, is a product used for cleaning or dusting electronic equipment and other sensitive devices that cannot be cleaned using water.

High-fructose corn syrup (HFCS), also known as glucose–fructose, isoglucose and glucose–fructose syrup, is a sweetener made from corn starch. As in the production of conventional corn syrup, the starch is broken down into glucose by enzymes. To make HFCS, the corn syrup is further processed by D-xylose isomerase to convert some of its glucose into fructose. HFCS was first marketed in the early 1970s by the Clinton Corn Processing Company, together with the Japanese Agency of Industrial Science and Technology, where the enzyme was discovered in 1965.
Tetrachloroethane may refer to either of two isomeric chemical compounds:
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being apart of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.
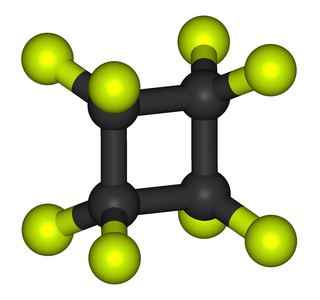
Octafluorocyclobutane, or perfluorocyclobutane, C4F8, is an organofluorine compound which enjoys several niche applications. Octafluorocyclobutane is a colourless gas and shipped as a liquefied gas. It is the perfluorinated analogue of cyclobutane whereby all C–H bonds are replaced with C–F bonds.
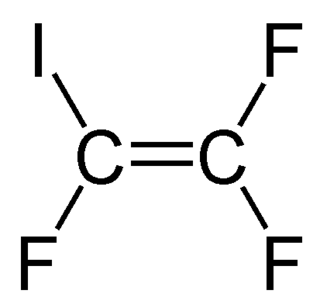
Iodotrifluoroethylene is the organofluorine compound with the formula C
2F
3I. It is a volatile colorless liquid.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

1,1,1,3,3-Pentafluoropropane (HFC-245fa) is a hydrofluorocarbon is a colorless gas used primarily for closed-cell spray foam insulation. HFC-245fa is also known as pentafluoropropane and by its chemical name 1,1,1,3,3-pentafluoropropane.
R-407A is a mixture of gasses used as a refrigerant. It is a zeotropic blend of difluoromethane (HFC-32), pentafluoroethane (HFC-125) and 1,1,1,2-tetrafluoroethane (HFC-134a). R-407A was developed as a close match to R-22's capacities and flow rates, making it well suited as an energy efficient retrofit for R-22 in medium and low temperature refrigeration systems for supermarket and food storage applications, but not for air conditioning systems or those with flooded evaporators. It must be used with synthetic oils. Its global warming potential is 2107.
R-407C is a mixture of hydrofluorocarbons used as a refrigerant. It is a zeotropic blend of difluoromethane (R-32), pentafluoroethane (R-125), and 1,1,1,2-tetrafluoroethane (R-134a). Difluoromethane serves to provide the heat capacity, pentafluoroethane decreases flammability, tetrafluoroethane reduces pressure. R-407C cylinders are colored burnt orange.
Fluorinated gases (F-gases) are chemical compounds containing fluorine that are gases near room temperature.
R134, R 134, or R-134 may refer to:










