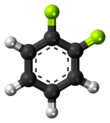
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. It is an isomer of another solvent, butanone.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, dihydroxybenzenes (benzenediols) are organic compounds in which two hydroxyl groups are substituted onto a benzene ring. These aromatic compounds are classed as phenols. There are three structural isomers: 1,2-dihydroxybenzene is commonly known as catechol, 1,3-dihydroxybenzene is commonly known as resorcinol, and 1,4-dihydroxybenzene is commonly known as hydroquinone.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent compound where R is hydrogen, is diazenylium.
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH) and NOBF4. The ClO−4 and BF−4 salts are slightly soluble in acetonitrile CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).
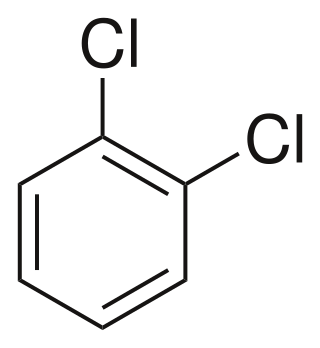
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.
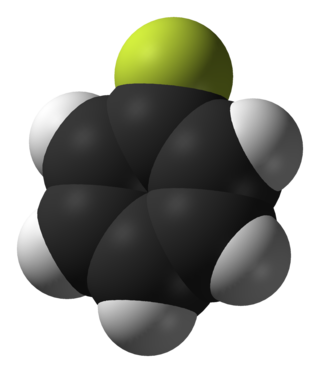
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.
(Benzene)chromium tricarbonyl is an organometallic compound with the formula Cr(C6H6)(CO)3. This yellow crystalline solid compound is soluble in common nonpolar organic solvents. The molecule adopts a geometry known as “piano stool” because of the planar arrangement of the aryl group and the presence of three CO ligands as "legs" on the chromium-bond axis.

Rhodocene is a chemical compound with the formula [Rh(C5H5)2]. Each molecule contains an atom of rhodium bound between two planar aromatic systems of five carbon atoms known as cyclopentadienyl rings in a sandwich arrangement. It is an organometallic compound as it has (haptic) covalent rhodium–carbon bonds. The [Rh(C5H5)2] radical is found above 150 °C (302 °F) or when trapped by cooling to liquid nitrogen temperatures (−196 °C [−321 °F]). At room temperature, pairs of these radicals join via their cyclopentadienyl rings to form a dimer, a yellow solid.

Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]−, indicating the presence of fluorinated aryl (ArF) groups. It is sometimes referred to as Kobayashi's anion in honour of Hiroshi Kobayashi who led the team that first synthesised it. More commonly it is affectionately nicknamed "BARF." The BARF ion is also abbreviated BArF24−, to distinguish it from the closely related BArF−
20, [(C6F5)4B]−. However, for a small group of chemists, the anion is abbreviated as TFPB otherwise, short for Tetrakis[3,5-bis(triFluoromethyl)Phenyl]Borate.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
A metal-centered cycloaddition is a subtype of the more general class of cycloaddition reactions. In such reactions "two or more unsaturated molecules unite directly to form a ring", incorporating a metal bonded to one or more of the molecules. Cycloadditions involving metal centers are a staple of organic and organometallic chemistry, and are involved in many industrially-valuable synthetic processes.
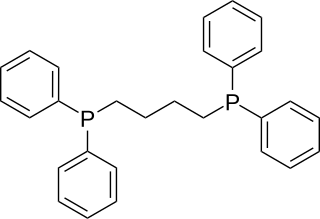
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.