L-Tyrosine or tyrosine or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.
Safrole is an organic compound with the formula CH2O2C6H3CH2CH=CH2. It is a colorless oily liquid, although impure samples can appear yellow. A member of the phenylpropanoid family of natural products, it is found in sassafras plants, among others. Small amounts are found in a wide variety of plants, where it functions as a natural antifeedant. Ocotea pretiosa, which grows in Brazil, and Sassafras albidum, which grows in eastern North America, are the main natural sources of safrole. It has a characteristic "sweet-shop" aroma.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body.

Sudan I, is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.

Paraxanthine, or 1,7-dimethylxanthine, is a dimethyl derivative of xanthine, structurally related to caffeine.

Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminate long-stored food and it causes different toxic effects, like nephrotoxic, hepatotoxic and cytotoxic effects. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.
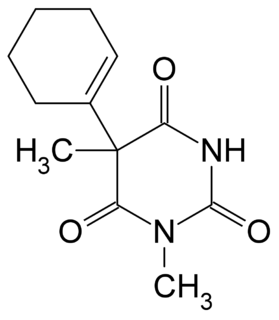
Hexobarbital or hexobarbitone, sold both in acid and sodium salt forms as Citopan, Evipan, and Tobinal, is a barbiturate derivative having hypnotic and sedative effects. It was used in the 1940s and 1950s as an agent for inducing anesthesia for surgery, as well as a rapid-acting, short-lasting hypnotic for general use, and has a relatively fast onset of effects and short duration of action. It was also used to murder women prisoners at Ravensbrück concentration camp. Modern barbiturates have largely supplanted the use of hexobarbital as an anesthetic, as they allow for better control of the depth of anesthesia. Hexobarbital is still used in some scientific research.

o-Toluidine (ortho-toluidine) is an organic compound with the chemical formula CH3C6H4NH2. It is the most important of the three isomeric toluidines. It is a colorless liquid although commercial samples are often yellowish. It is a precursor to the herbicides metolachlor and acetochlor.
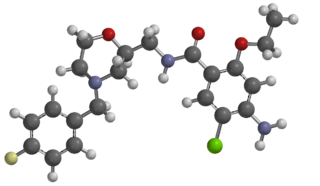
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
Arsenic biochemistry refers to biochemical processes that can use arsenic or its compounds, such as arsenate. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compounds are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organisms. This pattern is general for other related elements, including selenium, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifers, potentially affecting many millions of people via biochemical processes.

MT-45 (IC-6) is an opioid analgesic drug invented in the 1970s by Dainippon Pharmaceutical Co. It is chemically a 1-substituted-4-(1,2-diphenylethyl)piperazine derivative, which is structurally unrelated to most other opioid drugs. Racemic MT-45 has around 80% the potency of morphine, with almost all opioid activity residing in the (S) enantiomer. It has been used as a lead compound from which a large family of potent opioid drugs have been developed, including full agonists, partial agonists, and antagonists at the three main opioid receptor subtypes. Fluorinated derivatives of MT-45 such as 2F-MT-45 are significantly more potent as μ-opioid receptor agonists, and one of its main metabolites 1,2-diphenylethylpiperazine also blocks NMDA receptors.

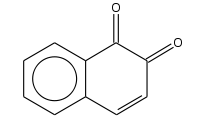
1,4-Naphthoquinone or para-naphthoquinone is an organic compound derived from naphthalene. It forms volatile yellow triclinic crystals and has a sharp odor similar to benzoquinone. It is almost insoluble in cold water, slightly soluble in petroleum ether, and more soluble in polar organic solvents. In alkaline solutions it produces a reddish-brown color. Vitamin K is a derivative of 1,4-naphthoquinone. It is a planar molecule with one aromatic ring fused to a quinone subunit. It is an isomer of 1,2-naphthoquinone.

9-Hydroxyoctadecadienoic acid has been used in the literature to designate either or both of two stereoisomer metabolites of the essential fatty acid, linoleic acid: 9(S)-hydroxy-10(E),12(Z)-octadecadienoic acid and 9(R)-hydroxy-10(E),12(Z)-octadecadienoic acid ; these two metabolites differ in having their hydroxy residues in the S or R configurations, respectively. The accompanying figure gives the structure for 9(S)-HETE. Two other 9-hydroxy linoleic acid derivatives occur in nature, the 10E,12E isomers of 9(S)-HODE and 9(R)-HODE viz., 9(S)-hydroxy-10E,12E-octadecadienoic acid and 9(R)-hydroxy-10E,12E-octadecadienoic acid ; these two derivatives have their double bond at carbon 12 in the E or trans configuration as opposed to the Z or cis configuration. The four 9-HODE isomers, particularly under conditions of oxidative stress, may form together in cells and tissues; they have overlapping but not identical biological activities and significances. Because many studies have not distinguished between the S and R stereoisomers and, particularly in identifying tissue levels, the two EE isomers, 9-HODE is used here when the isomer studied is unclear.

Geniposide is a bioactive iridoid glycoside that is found in a wide variety of medicinal herbs, such as Gardenia jasminoides (fruits) . Geniposide shows several pharmacological effects including neuroprotective, antidiabetic, hepatoprotective, anti-inflammatory, analgesic, antidepressant-like, cardioprotective, antioxidant, immune-regulatory, antithrombotic and antitumoral activity. These pharmacology benefits arise through the modulating action of geniposide on several proteins and genes that are associated with inflammatory and oxidative stress processes.

In chemistry, an arene oxide is an epoxide of an arene. Two important families of arene oxides are benzene oxides and naphthalene oxides as these are intermediates in the oxidative degradation of benzene and naphthalene, two common pollutants. Benzopyrene is also converted to an epoxide, (+)-benzo[a]pyrene-7,8-epoxide.
Naphthalene poisoning is a form of poisoning that occurs when naphthalene is ingested. Severe poisoning can result in haemolytic anaemia. Naphthalene was introduced in 1841 by Rossbach as an antiseptic to counteract typhoid fever. Although naphthalene was widely used industrially, only nine cases of poisoning have been reported since 1947. As a result, the condition has limited coverage within medical journals.