 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1,3,5-Trinitrobenzene | |
| Other names sym-Trinitrobenzene | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.002.502 |
PubChem CID | |
| UNII | |
| UN number | 0388 |
CompTox Dashboard (EPA) | |
| |
| Properties | |
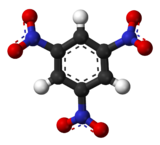
| C6H3N3O6 | |
| Molar mass | 213.105 g·mol−1 |
| Density | 1.76 g/cm3 |
| Melting point | 123.2 °C (253.8 °F; 396.3 K) |
| Boiling point | 315 °C (599 °F; 588 K) |
| 330 mg/L | |
| −74.55·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1,3,5-Trinitrobenzene is one of three isomers of trinitrobenzene with the formula C6H3(NO2)3. A pale yellow solid, the compound is highly explosive. [2]
