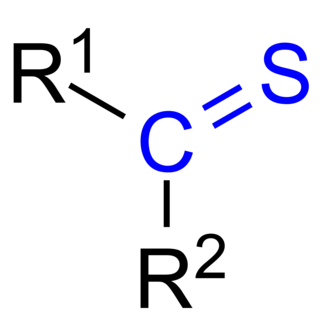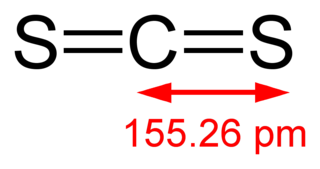
Carbon disulfide is an inorganic compound with the chemical formula CS2 and structure S=C=S. It is a colorless, flammable, neurotoxic liquid that is used as a building block in organic synthesis. Pure carbon disulfide has a pleasant, ether- or chloroform-like odor, but commercial samples are usually yellowish and are typically contaminated with foul-smelling impurities.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2·nH2O, with x ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Sodium dithionite is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.
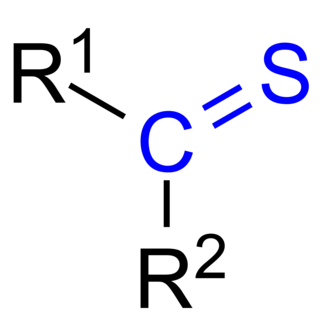
In organic chemistry, thioketones are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Thus the simplest thioketone is thioacetone, the sulfur analog of acetone. Unhindered alkylthioketones typically tend to form polymers or rings.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.
Tm is an abbreviation for anionic tridentate ligand based on three imidazole-2-thioketone groups bonded to a borohydride center. They are examples of scorpionate ligands. Various ligands in this family are known, differing in what substituents are on the imidazoles. The most common is TmMe, which has a methyl group on the nitrogen. It is easily prepared by the reaction of molten methimazole (1-methylimidazole-2-thione) with sodium borohydride, giving the sodium salt of the ligand. Salts of the TmMe anion are known also for lithium and potassium. Other alkyl- and aryl-group variations are likewise named TmR according to those groups.

Tetrathiafulvalene (TTF) is an organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.

Anethole trithione, anetholtrithione, or anetholtrithion (JAN) is a drug used in the treatment of dry mouth. It is listed in the U.S. National Cancer Institute's Dictionary of Cancer Terms as being studied in the treatment of cancer. Anethole trithione is an organosulfur compound, specifically, a dithiole-thione derivative.
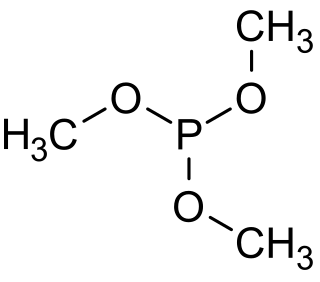
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

Galactoside 3(4)-L-fucosyltransferase is an enzyme that in humans is encoded by the FUT3 gene.

Alpha-(1,3)-fucosyltransferase is an enzyme that in humans is encoded by the FUT5 gene.
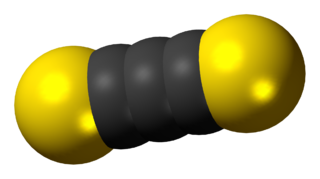
Carbon subsulfide is an organic, sulfur-containing chemical compound with the formula C3S2 and structure S=C=C=C=S. This deep red liquid is immiscible with water but soluble in organic solvents. It readily polymerizes at room temperature to form a hard black solid.

Anthony Joseph Arduengo III is Professor of the Practice at the Georgia Institute of Technology, Saxon Professor Emeritus of Chemistry at the University of Alabama, adjunct professor at the Institute for Inorganic Chemistry of Braunschweig University of Technology in Germany, and co-founder of the StanCE coalition for sustainable chemistry based on woody biomass. He is notable for his work on chemical compounds with unusual valency, especially in the field of stable carbene research.

In organic chemistry, a dithiol is a type of organosulfur compound with two thiol functional groups. Their properties are generally similar to those of monothiols in terms of solubility, odor, and volatility. They can be classified according to the relative location of the two thiol groups on the organic backbone.
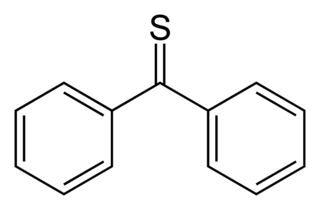
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air back to benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.

In organic chemistry, TADDOL is an acronym for α,α,α',α'-tetraaryl-2,2-disubstituted 1,3-dioxolane-4,5-dimethanol. These compounds are easily accessed and are often used as chiral auxiliaries.
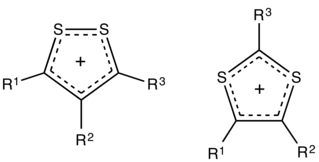
Dithiolium salts are compounds of the formula [(RC)3S2]+X− (R = H, alkyl, aryl, etc.). These salts consist of a planar organic cation with a variety of anions such as halides. The five-membered ring cations are observed in either of two isomers, 1,2- and 1,3-dithiolium cations. These cations differ with respect to the relative positions of the pair of sulfur atoms. Both isomers feature a planar ring, which is aromatic owing to the presence of 6π electrons. For example, the 1,2-ditholium ring can be represented as an allyl cation of the three carbons, with each sulfur atom donating one of its lone pairs of electrons to give a total of three pairs.

Sodium 1,3-dithiole-2-thione-4,5-dithiolate is the organosulfur compound with the formula Na2C3S5, abbreviated Na2dmit. It is the sodium salt of the conjugate base of the 4,5-bis(sulfanyl)-1,3-dithiole-2-thione. The salt is a precursor to dithiolene complexes and tetrathiafulvalenes.
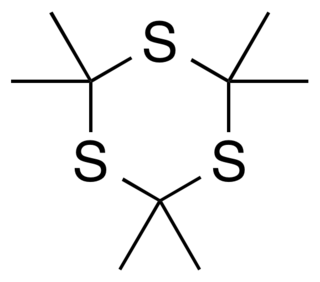
Trithioacetone (2,2,4,4,6,6-hexamethyl-1,3,5-trithiane) is an organic chemical with formula C
9H
18S
3. Its covalent structure is [–C(CH
3)
2–S–]
3, that is, a six-membered ring of alternating carbon and sulfur atoms, with two methyl groups attached to each carbon. It can be viewed as a derivative of 1,3,5-trithiane, with methyl-group substituents for all of the hydrogen atoms in that parent structure.

![Structure of the anion [Zn(dmit)2] , featuring two 1,3-dithiole-4,5-dithiolate ligands complexed to zinc. DOQXOW.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/28/DOQXOW.png/220px-DOQXOW.png)