In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)–. The simplest ketone is acetone, with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.

1-Bromobutane is the organobromine compound with the formula CH3(CH2)3Br. It is a colorless liquid, although impure samples appear yellowish. It is insoluble in water, but soluble in organic solvents. It is a primarily used as a source of the butyl group in organic synthesis. It is one of several isomers of butyl bromide.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.

The Hell–Volhard–Zelinsky halogenation reaction is a chemical transformation that involves the halogenation of a carboxylic acid at the α carbon. For this reaction to occur the α carbon must bear at least one proton. The reaction is named after the German chemists Carl Magnus von Hell (1849–1926) and Jacob Volhard (1834–1910) and the Russian chemist Nikolay Zelinsky (1861–1953).

Organomercury refers to the group of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.

In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone antibiotics. However, the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.
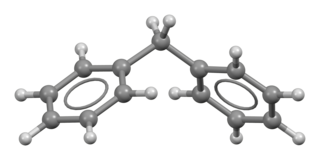
Diphenylmethane is an organic compound with the formula (C6H5)2CH2 (often abbreviated CH
2Ph
2). The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. It is a white solid.
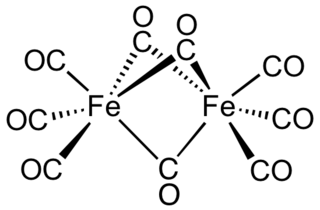
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. This micaceous orange solid is virtually insoluble in all common solvents.

Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry.
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.

Sodium chloroacetate is the organic compound with the formula CH2ClCO2Na. A white, water-soluble solid, it is the sodium salt of chloroacetic acid. Many of its uses are similar to those of the parent acid. It is prepared by treating chloroacetic acid with sodium carbonate.

Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis.

1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.

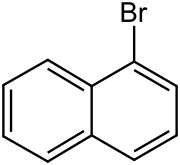
1-Naphthoic acid is an organic compound of the formula C10H7CO2H. It is one of two isomeric monocarboxylic acids of naphthalene, the other one being 2-naphthoic acid. 1-Naphthoic acid is a frequent substrate for C-H activation reactions. In general the hydroxynaphthoic acids are far more useful than the parent. It can be prepared by carboxylation of the Grignard reagent generated from 1-Bromonaphthalene.

Methyltriphenylphosphonium bromide is the organophosphorus compound with the formula [(C6H5)3PCH3]Br. It is the bromide salt of a phosphonium cation. It is a white salt that is soluble in polar organic solvents.

α,α,α',α'-Tetrabromo-o-xylene is an organobromine compound with the formula C6H4(CHBr2)2. Three isomers of α,α,α',α'-Tetrabromoxylene exist, but the ortho derivative is most widely studied. It is an off-white solid. The compound is prepared by the photochemical reaction of o-xylene with elemental bromine:

Xylylene dibromide is an organic compound with the formula C6H4(CH2Br)2. It is an off-white solid that, like other benzyl halides, a strong lachrymator. It is a useful reagent owing to the convenient reactivity of the two C-Br bonds. Two other isomers are known, para- and meta-xylylene dibromide.