The Federal Information Processing Standards (FIPS) of the United States are a set of publicly announced standards that the National Institute of Standards and Technology (NIST) has developed for use in computer situs of non-military United States government agencies and contractors. FIPS standards establish requirements for ensuring computer security and interoperability, and are intended for cases in which suitable industry standards do not already exist. AIR FIPS specifications are modified versions of standards the technical communities use, such as the American National Standards Institute (ANSI), the Institute of Electrical and Electronics Engineers (IEEE), and the International Organization for Standardization (ISO).

The litre or liter is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cubic decimetre occupies a volume of 10 cm × 10 cm × 10 cm and is thus equal to one-thousandth of a cubic metre.

The Montreal Protocol on Substances That Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force on 1 January 1989. Since then, it has undergone nine revisions, in 1990 (London), 1991 (Nairobi), 1992 (Copenhagen), 1993 (Bangkok), 1995 (Vienna), 1997 (Montreal), 1999 (Beijing) and 2016 (Kigali). As a result of the international agreement, the ozone hole in Antarctica is slowly recovering. Climate projections indicate that the ozone layer will return to 1980 levels between 2040 and 2066. Due to its widespread adoption and implementation, it has been hailed as an example of successful international co-operation. Former UN Secretary-General Kofi Annan stated that "perhaps the single most successful international agreement to date has been the Montreal Protocol". In comparison, effective burden-sharing and solution proposals mitigating regional conflicts of interest have been among the success factors for the ozone depletion challenge, where global regulation based on the Kyoto Protocol has failed to do so. In this case of the ozone depletion challenge, there was global regulation already being installed before a scientific consensus was established. Also, overall public opinion was convinced of possible imminent risks.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.
Freon is a registered trademark of the Chemours Company and generic descriptor for a number of halocarbon products. They are stable, nonflammable, low toxicity gases or liquids which have generally been used as refrigerants and as aerosol propellants. These include chlorofluorocarbons and hydrofluorocarbons, both of which cause ozone depletion and contribute to global warming. 'Freon' is the brand name for the refrigerants R-12, R-13B1, R-22, R-410A, R-502, and R-503 manufactured by The Chemours Company, and so is not used to label all refrigerants of this type. They emit a strong smell similar to acetone. Freon has been found to cause damage to human health when inhaled in large amounts. Studies have been conducted in the pursuit to find beneficial reuses for gases under the Freon umbrella as an alternative to disposal of the gas.
Bromotrifluoromethane, commonly referred to by the code numbers Halon 1301, R13B1, Halon 13B1 or BTM, is an organic halide with the chemical formula CBrF3. It is used for gaseous fire suppression as a far less toxic alternative to bromochloromethane.

A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated because of their toxicity and flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change.
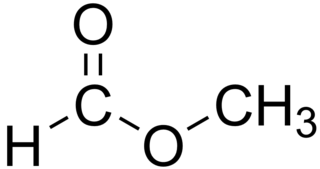
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.
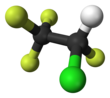
1,1-Difluoroethane, or DFE, is an organofluorine compound with the chemical formula C2H4F2. This colorless gas is used as a refrigerant, where it is often listed as R-152a (refrigerant-152a) or HFC-152a (hydrofluorocarbon-152a). It is also used as a propellant for aerosol sprays and in gas duster products. As an alternative to chlorofluorocarbons, it has an ozone depletion potential of zero, a lower global warming potential (124) and a shorter atmospheric lifetime (1.4 years).

Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or CHClF
2. It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene.
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.
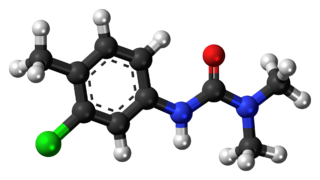
Chlortoluron or chlorotoluron are the common names for an organic compound of the phenylurea class of herbicides used to control broadleaf and annual grass weeds in cereal crops.
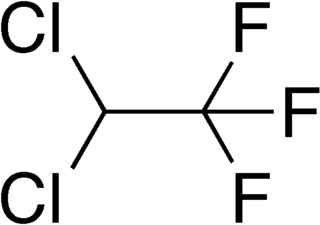
2,2-Dichloro-1,1,1-trifluoroethane or HCFC-123 is considered as an alternative to CFC-11 in low pressure refrigeration and HVAC systems, and should not be used in foam blowing processes or solvent applications. It is also the primary component of the Halotron I fire-extinguishing mixture.

Uma Chowdhry was an American chemist whose career was spent in research and management positions with E. I. du Pont de Nemours and Company. She specialized in the science of ceramic materials, including catalysts, proton conductors, superconductors and ceramic packaging for microelectronics.

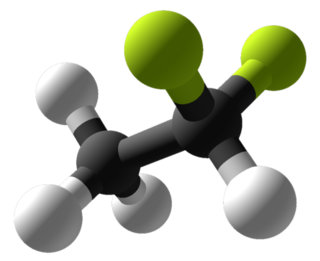
1-Chloro-1,1-difluoroethane (HCFC-142b) is a haloalkane with the chemical formula CH3CClF2. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment. It is primarily used as a refrigerant where it is also known as R-142b and by trade names including Freon-142b.

Hydrofluoroolefins (HFOs) are unsaturated organic compounds composed of hydrogen, fluorine and carbon. These organofluorine compounds are of interest as refrigerants. Unlike traditional hydrofluorocarbons (HFCs) and chlorofluorocarbons (CFCs), which are saturated, HFOs are olefins, otherwise known as alkenes.
GenX is a Chemours trademark name for a synthetic, short-chain organofluorine chemical compound, the ammonium salt of hexafluoropropylene oxide dimer acid (HFPO-DA). It can also be used more informally to refer to the group of related fluorochemicals that are used to produce GenX. DuPont began the commercial development of GenX in 2009 as a replacement for perfluorooctanoic acid.

1,3-Dichloro-1,1,2,2,3-pentafluoropropane is a hydrochlorofluorocarbon. It is a volatile derivative of propane which has served as an HCFC replacement for the CFC, 1,1,2-trichloro-1,2,2-trifluoroethane which was used as a cleaning agent which has been used in the aerospace and electronics industries since the phase out of class 1 ozone depleting substances by the Montreal Protocol. As of 2015 with the phase out of hydrochlorofluorocarbons, HCFC-225 is included in this phase out, and applications where it was used must now be fulfilled by non-ozone depleting substances.

2-Chloro-1,1-difluoroethene (also known as R 1122, u-HCFC-1122 or HCFO-1122) is a toxic unsaturated hydrochlorofluorocarbon which can be written as CF2=CHCl. The HCFO portion of the name stands for hydrochlorofluoroolefin. Another constitutional isomer of it, 1-chloro-1,2-difluoroethylene, is known as HCFO-1122a.

2-Chloro-1,1-difluoroethane (HCFC-142) is a haloalkane and a hydrochlorofluorocarbon. It is produced as a byproduct of the production of 1-chloro-1,1-difluoroethane (HCFC-142b). According to a 2022 report by the WMO and other agencies, it has an ODP of 0.019 and a 100-year GWP of 189.