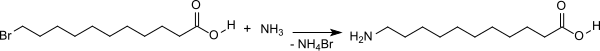In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Nylon is a family of synthetic polymers with amide backbones, usually linking aliphatic or semi-aromatic groups.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Castor oil is a vegetable oil pressed from castor beans, the seeds of the plant Ricinus communis. The seeds are 40 to 60 percent oil. It is a colourless or pale yellow liquid with a distinct taste and odor. Its boiling point is 313 °C (595 °F) and its density is 0.961 g/cm3. It includes a mixture of triglycerides in which about 90 percent of fatty acids are ricinoleates. Oleic acid and linoleic acid are the other significant components.
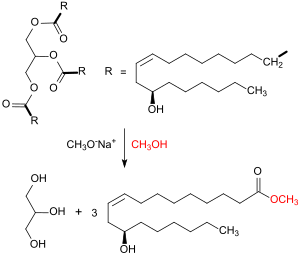
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases.
A polyamide is a polymer with repeating units linked by amide bonds.

In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally-occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups. The general molecular formula for dicarboxylic acids can be written as HO2C−R−CO2H, where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids.
Enanthic acid, also called heptanoic acid, is an organic compound composed of a seven-carbon chain terminating in a carboxylic acid functional group. It is a colorless oily liquid with an unpleasant, rancid odor. It contributes to the odor of some rancid oils. It is slightly soluble in water, but very soluble in ethanol and ether. Salts and esters of enanthic acid are called enanthates or heptanoates.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
Kolliphor EL, formerly known as Cremophor EL, is the registered trademark of BASF Corp. for its version of polyethoxylated castor oil. It is prepared by reacting 35 moles of ethylene oxide with each mole of castor oil. The resulting product is a mixture : the major component is the material in which the hydroxyl groups of the castor oil triglyceride have been ethoxylated with ethylene oxide to form polyethylene glycol ethers. Minor components are the polyethyelene glycol esters of ricinoleic acid, polyethylene glycols and polyethylene glycol ethers of glycerol. Kolliphor EL is a synthetic, nonionic surfactant used to stabilize emulsions of nonpolar materials in water.

Polyglycerol polyricinoleate (PGPR), E476, is an emulsifier made from glycerol and fatty acids. In chocolate, compound chocolate and similar coatings, PGPR is mainly used with another substance like lecithin to reduce viscosity. It is used at low levels, and works by decreasing the friction between the solid particles in molten chocolate, reducing the yield stress so that it flows more easily, approaching the behaviour of a Newtonian fluid. It can also be used as an emulsifier in spreads and in salad dressings, or to improve the texture of baked goods. It is made up of a short chain of glycerol molecules connected by ether bonds, with ricinoleic acid side chains connected by ester bonds.
Undecylenic acid is an organic compound with the formula CH2=CH(CH2)8CO2H. It is an unsaturated fatty acid. It is a colorless oil. Undecylenic acid is mainly used for the production of Nylon-11 and in the treatment of fungal infections of the skin, but it is also a precursor in the manufacture of many pharmaceuticals, personal hygiene products, cosmetics, and perfumes. Salts and esters of undecylenic acid are known as undecylenates.

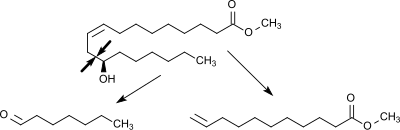
Ricinoleic acid, formally called 12-hydroxy-9-cis-octadecenoic acid, is a fatty acid. It is an unsaturated omega-9 fatty acid and a hydroxy acid. It is a major component of the seed oil obtained from the seeds of castor plant, the plant that produces ricin. It is also found in the sclerotium of ergot. About 90% of the fatty acid content in castor oil is the ricinolein.
Nylon 11 or Polyamide 11 is a polyamide, bioplastic and a member of the nylon family of polymers produced by the polymerization of 11-aminoundecanoic acid. It is produced from castor beans by Arkema under the trade name Rilsan.
Natural oil polyols, also known as NOPs or biopolyols, are polyols derived from vegetable oils by several different techniques. The primary use for these materials is in the production of polyurethanes. Most NOPs qualify as biobased products, as defined by the United States Secretary of Agriculture in the Farm Security and Rural Investment Act of 2002.
Heptanal or heptanaldehyde is an alkyl aldehyde. It is a colourless liquid with a strong fruity odor, which is used as precursor to components in perfumes and lubricants.

2-Methylglutaronitrile is the organic compound with the formula NCCH2CH2CH(CH3)CN. This dinitrile is obtained in the large-scale synthesis of adiponitrile. It is a colorless liquid with an unpleasant odor. It is the starting compound for the vitamin nicotinamide and for the diester dimethyl-2-methylglutarate and the ester amide methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, which are promoted as green solvents. 2-Methylglutaronitrile is chiral but is mainly encountered as the racemate. It is also used to make Dytek A.
Jasminaldehyde is a fine chemical used as an aroma compound in perfumes. It is responsible for jasmine's characteristic scent.
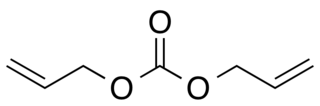
Diallyl carbonate (DAC) is a colorless liquid with a pungent odor. Its structure contains allyl groups and a functional carbonate group. The presence of double bonds in the allyl groups makes it reactive in various chemical processes. This compound plays a key role in the production of polymers, including polycarbonates and polyurethanes. Diallyl carbonate is soluble in ethanol, methanol, toluene, and chloroform. Diallyl carbonate reacts with amines, alcohols, and thiols.