
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula RC(=O)NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.

In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.

In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula C6H4(OH)2. It is the ortho isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It was first discovered by destructive distillation of the plant extract catechin. About 20,000 tonnes of catechol are now synthetically produced annually as a commodity organic chemical, mainly as a precursor to pesticides, flavors, and fragrances.

Cyanate is an anion with the structural formula [O=C=N]−, usually written OCN−. It also refers to any salt containing it, such as ammonium cyanate.
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, being able to form oximes; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.

Metal–organic frameworks (MOFs) are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC).

Croconic acid or 4,5-dihydroxycyclopentenetrione is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.

Chloranilic acid is an organic compound with the chemical formula C6Cl2O2(OH)2. It is a red-orange solid. The compound is obtained by hydrolysis of chloranil:
A hydroxynaphthoquinone is any of several organic compounds that can be viewed as derivatives of a naphthoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
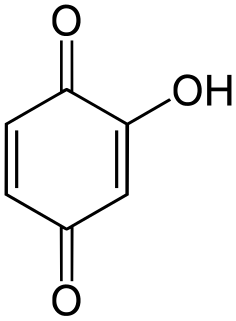
Hydroxyquinone often refers to a hydroxybenzoquinone, any organic compound with formula C
6H
4O
3 which can be viewed as a derivative of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH). When unqualified, the terms usually mean specifically the compound 2-hydroxy-1,4-benzoquinone, derived from 1,4-benzoquinone or para-benzoquinone.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).

Dimethylol ethyleneurea is an organic compound derived from formaldehyde and urea. It is a colourless solid that is used for treating cellulose-based heavy fabrics to inhibit wrinkle formation. Dimethylol ethylene urea (DMEU) bonds with the hydroxyl groups present in long cellulose chains and prevents the formation hydrogen bonding between the chains, the primary cause of wrinkling. This treatment produces permanently wrinkle-resistant fabrics and is different from the effects achieved from using fabric softeners.















