| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Hydroxycyclohexa-2,5-diene-1,4-dione | |||
| Other names 2-Hydroxy-p-benzoquinone | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
PubChem CID | |||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H4O3 | |||
| Molar mass | 124.1 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
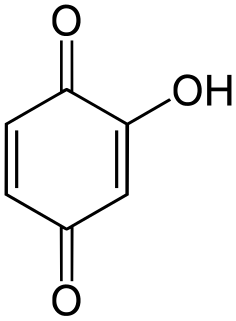
Hydroxy-1,4-benzoquinone, also called hydroxy-para-benzoquinone, is an organic compound with formula C
6H
4O
3, formally derived from 1,4-Benzoquinone by replacing one hydrogen atom with a hydroxyl (OH) group. It is one of three hydroxybenzoquinone isomers and one of the simplest hydroxyquinones.

1,4-Benzoquinone, commonly known as para-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic or formaldehyde. This six-membered ring compound is the oxidized derivative of 1,4-hydroquinone. The molecule is multifunctional: it exhibits properties of a ketone, forming an oxime; an oxidant, forming the dihydroxy derivative; and an alkene, undergoing addition reactions, especially those typical for α,β-unsaturated ketones. 1,4-Benzoquinone is sensitive toward both strong mineral acids and alkali, which cause condensation and decomposition of the compound.
Hydrogen is a chemical element with symbol H and atomic number 1. With a standard atomic weight of 1.008, hydrogen is the lightest element in the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass. Non-remnant stars are mainly composed of hydrogen in the plasma state. The most common isotope of hydrogen, termed protium, has one proton and no neutrons.
A hydroxybenzoquinone is any of several organic compounds that can be viewed as derivatives of a benzoquinone through replacement of one hydrogen atom (H) by a hydroxyl group (-OH).
The compound is often called 2-hydroxy-1,4-benzoquinone, but the "2-" prefix is superfluous since there is no other hydroxy derivative of 1,4-benzoquinone. The IUPAC name is 2-hydroxycyclohexa-2,5-diene-1,4-dione.
It is formed by the reaction of 1,4-benzoquinone with hydrogen peroxide and is a byproduct of the metabolism of phenols, such as 1,2,4-benzenetriol. [1] The enzyme 1,2,4-benzenetriol dehydrogenase catalyzes the conversion of 1,2,4-benzenetriol to 2-hydroxy-1,4-benzoquinone, and the enzyme hydroxybenzoquinone reductase catalyzes the reverse reaction. The enzyme 2-hydroxy-1,4-benzoquinone-2-reductase converts it to 1,4-benzoquinone.

Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxy group (−OH). It is mildly acidic and requires careful handling due to its propensity for causing chemical burns.

Enzymes are macromolecular biological catalysts. Enzymes accelerate chemical reactions. The molecules upon which enzymes may act are called substrates and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.